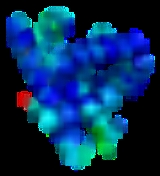
Biomolecule
Overview
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
that is produced by a living organism
Organism
In biology, an organism is any contiguous living system . In at least some form, all organisms are capable of response to stimuli, reproduction, growth and development, and maintenance of homoeostasis as a stable whole.An organism may either be unicellular or, as in the case of humans, comprise...
, including large polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
ic molecules such as protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s, polysaccharide
Polysaccharide
Polysaccharides are long carbohydrate molecules, of repeated monomer units joined together by glycosidic bonds. They range in structure from linear to highly branched. Polysaccharides are often quite heterogeneous, containing slight modifications of the repeating unit. Depending on the structure,...
s, lipid
Lipid
Lipids constitute a broad group of naturally occurring molecules that include fats, waxes, sterols, fat-soluble vitamins , monoglycerides, diglycerides, triglycerides, phospholipids, and others...
s, and nucleic acid
Nucleic acid
Nucleic acids are biological molecules essential for life, and include DNA and RNA . Together with proteins, nucleic acids make up the most important macromolecules; each is found in abundance in all living things, where they function in encoding, transmitting and expressing genetic information...
s as well as small molecule
Small molecule
In the fields of pharmacology and biochemistry, a small molecule is a low molecular weight organic compound which is by definition not a polymer...
s such as primary metabolite
Metabolite
Metabolites are the intermediates and products of metabolism. The term metabolite is usually restricted to small molecules. A primary metabolite is directly involved in normal growth, development, and reproduction. Alcohol is an example of a primary metabolite produced in large-scale by industrial...
s, secondary metabolites, and natural product
Natural product
A natural product is a chemical compound or substance produced by a living organism - found in nature that usually has a pharmacological or biological activity for use in pharmaceutical drug discovery and drug design...
s. A more general name for this class of molecules is a biogenic substance
Biogenic substance
A biogenic substance is a substance produced by life processes. It may be either constituents, or secretions, of plants or animals. A more specific name for these substances is biomolecules.-Examples:...
.
A diverse range of biomolecules exist, including:
- Small moleculeSmall moleculeIn the fields of pharmacology and biochemistry, a small molecule is a low molecular weight organic compound which is by definition not a polymer...
s:- LipidLipidLipids constitute a broad group of naturally occurring molecules that include fats, waxes, sterols, fat-soluble vitamins , monoglycerides, diglycerides, triglycerides, phospholipids, and others...
s, phospholipidPhospholipidPhospholipids are a class of lipids that are a major component of all cell membranes as they can form lipid bilayers. Most phospholipids contain a diglyceride, a phosphate group, and a simple organic molecule such as choline; one exception to this rule is sphingomyelin, which is derived from...
s, glycolipidGlycolipidGlycolipids are lipids with a carbohydrate attached. Their role is to provide energy and also serve as markers for cellular recognition.-Metabolism:...
s, sterolSterolSterols, also known as steroid alcohols, are a subgroup of the steroids and an important class of organic molecules. They occur naturally in plants, animals, and fungi, with the most familiar type of animal sterol being cholesterol...
s, glycerolipids - VitaminVitaminA vitamin is an organic compound required as a nutrient in tiny amounts by an organism. In other words, an organic chemical compound is called a vitamin when it cannot be synthesized in sufficient quantities by an organism, and must be obtained from the diet. Thus, the term is conditional both on...
s - HormoneHormoneA hormone is a chemical released by a cell or a gland in one part of the body that sends out messages that affect cells in other parts of the organism. Only a small amount of hormone is required to alter cell metabolism. In essence, it is a chemical messenger that transports a signal from one...
s, neurotransmitterNeurotransmitterNeurotransmitters are endogenous chemicals that transmit signals from a neuron to a target cell across a synapse. Neurotransmitters are packaged into synaptic vesicles clustered beneath the membrane on the presynaptic side of a synapse, and are released into the synaptic cleft, where they bind to...
s - MetaboliteMetaboliteMetabolites are the intermediates and products of metabolism. The term metabolite is usually restricted to small molecules. A primary metabolite is directly involved in normal growth, development, and reproduction. Alcohol is an example of a primary metabolite produced in large-scale by industrial...
s
- Lipid
- MonomerMonomerA monomer is an atom or a small molecule that may bind chemically to other monomers to form a polymer; the term "monomeric protein" may also be used to describe one of the proteins making up a multiprotein complex...
s, oligomerOligomerIn chemistry, an oligomer is a molecule that consists of a few monomer units , in contrast to a polymer that, at least in principle, consists of an unlimited number of monomers. Dimers, trimers, and tetramers are oligomers. Many oils are oligomeric, such as liquid paraffin...
s and polymerPolymerA polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
s:
Nucleosides are molecules formed by attaching a nucleobase
Nucleobase
Nucleobases are a group of nitrogen-based molecules that are required to form nucleotides, the basic building blocks of DNA and RNA. Nucleobases provide the molecular structure necessary for the hydrogen bonding of complementary DNA and RNA strands, and are key components in the formation of stable...
to a ribose
Ribose
Ribose is an organic compound with the formula C5H10O5; specifically, a monosaccharide with linear form H––4–H, which has all the hydroxyl groups on the same side in the Fischer projection....
ring.
Discussions

