
Nitrous acid
Encyclopedia
Nitrous acid is a weak and monobasic acid
known only in solution
and in the form of nitrite
salts.
Nitrous acid is used to make diazides
from amines; this occurs by nucleophilic attack of the amine onto the nitrite
, reprotonation by the surrounding solvent, and double-elimination of water. The diazide can then be liberated to give a carbene
or carbenoid
.
indicate it is more stable
by around 2.3 kJ mol−1.
, NO2− are carefully acidified, a light blue solution of nitrous acid is produced. Free nitrous acid is unstable and decomposes rapidly.
Nitrogen dioxide
disproportionates into nitric acid
and nitrous acid in aqueous solution:
In warm or concentrated solutions, the overall reaction amounts to production of nitric acid, water, and nitric oxide:
where Ar is an aryl
group.
Such salts are widely used in organic synthesis
, e.g., for the Sandmeyer reaction
and in the preparation azo dyes, brightly-colored compounds that are the basis of a qualitative test for aniline
s. Nitrous acid is used to destroy toxic and potentially-explosive sodium azide
. For most purposes, nitrous acid is usually formed in situ by the action of mineral acid on sodium nitrite
:
Reaction with two α-hydrogen atoms in ketone
s creates oxime
s, which may be further oxidized to a carboxylic acid, or reduced to form amines. This process is used in the commercial production of adipic acid
.
Nitrous acid reacts rapidly with aliphatic alcohols
to produce alkyl nitrites
, which are potent vasodilators
:
2CH-CH2-CH2-OH + HNO2 → (CH3)2CH-CH2-CH2-ONO + H2O
budget of the lower atmosphere
: the troposphere
. The heterogeneous reaction of nitrogen monoxide (NO) and water produces nitrous acid. When this reaction takes place on the surface of atmospheric aerosol
s, product readily photolyses to hydroxyl
radical
s.
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
known only in solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
and in the form of nitrite
Nitrite
The nitrite ion has the chemical formula NO2−. The anion is symmetric with equal N-O bond lengths and a O-N-O bond angle of ca. 120°. On protonation the unstable weak acid nitrous acid is produced. Nitrite can be oxidised or reduced, with product somewhat dependent on the oxidizing/reducing agent...
salts.
Nitrous acid is used to make diazides
Diazo
Diazo refers to a type of organic compound called diazo compound that has two linked nitrogen atoms as a terminal functional group. The general formula is R2C=N2. The simplest example of a diazo compound is diazomethane...
from amines; this occurs by nucleophilic attack of the amine onto the nitrite
Nitrite
The nitrite ion has the chemical formula NO2−. The anion is symmetric with equal N-O bond lengths and a O-N-O bond angle of ca. 120°. On protonation the unstable weak acid nitrous acid is produced. Nitrite can be oxidised or reduced, with product somewhat dependent on the oxidizing/reducing agent...
, reprotonation by the surrounding solvent, and double-elimination of water. The diazide can then be liberated to give a carbene
Carbene
In chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
or carbenoid
Carbenoid
In chemistry a carbenoid is a reactive intermediate that shares reaction characteristics with a carbene. In the Simmons-Smith reaction the carbenoid intermediate is a zinc / iodine complex that takes the form of...
.
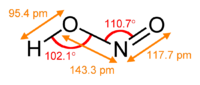
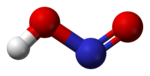
Structure
In the gas phase, the planar nitrous acid molecule can adopt both a cis and a trans form. The trans form predominates at room temperature, and IR measurementsInfrared spectroscopy
Infrared spectroscopy is the spectroscopy that deals with the infrared region of the electromagnetic spectrum, that is light with a longer wavelength and lower frequency than visible light. It covers a range of techniques, mostly based on absorption spectroscopy. As with all spectroscopic...
indicate it is more stable
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
by around 2.3 kJ mol−1.
 |
 |
 |
(from the microwave spectrum Rotational spectroscopy Rotational spectroscopy or microwave spectroscopy studies the absorption and emission of electromagnetic radiation by molecules associated with a corresponding change in the rotational quantum number of the molecule... ) |
Ball-and-stick model In chemistry, the ball-and-stick model is a molecular model of a chemical substance which is to display both the three-dimensional position of the atoms and the bonds between them... of the trans form |
Preparation
When cold, dilute solutions of nitrite ionNitrite
The nitrite ion has the chemical formula NO2−. The anion is symmetric with equal N-O bond lengths and a O-N-O bond angle of ca. 120°. On protonation the unstable weak acid nitrous acid is produced. Nitrite can be oxidised or reduced, with product somewhat dependent on the oxidizing/reducing agent...
, NO2− are carefully acidified, a light blue solution of nitrous acid is produced. Free nitrous acid is unstable and decomposes rapidly.
Decomposition
In anything other than very dilute, cold solutions, nitrous acid rapidly decomposes into nitrogen dioxide, nitric oxide, and water:- 2 HNO2 → NO2 + NO + H2O
Nitrogen dioxide
Nitrogen dioxide
Nitrogen dioxide is the chemical compound with the formula it is one of several nitrogen oxides. is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year. This reddish-brown toxic gas has a characteristic sharp, biting odor and is a prominent...
disproportionates into nitric acid
Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
and nitrous acid in aqueous solution:
- 2 NO2 + H2O → HNO3 + HNO2
In warm or concentrated solutions, the overall reaction amounts to production of nitric acid, water, and nitric oxide:
- 3 HNO2 → HNO3 + 2 NO + H2O
Chemistry
Nitrous acid is used to prepare diazonium salts:- HNO2 + ArNH2 + H+ → ArN2+ + 2 H2O
where Ar is an aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
group.
Such salts are widely used in organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
, e.g., for the Sandmeyer reaction
Sandmeyer reaction
The Sandmeyer reaction is a chemical reaction used to synthesize aryl halides from aryl diazonium salts. It is named after the Swiss chemist Traugott Sandmeyer....
and in the preparation azo dyes, brightly-colored compounds that are the basis of a qualitative test for aniline
Aniline
Aniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Being a precursor to many industrial chemicals, its main use is in the manufacture of precursors to polyurethane...
s. Nitrous acid is used to destroy toxic and potentially-explosive sodium azide
Sodium azide
Sodium azide is the inorganic compound with the formula NaN3. This colourless azide salt is the gas-forming component in many car airbag systems. It is used for the preparation of other azide compounds. It is an ionic substance and is highly soluble in water. It is extremely...
. For most purposes, nitrous acid is usually formed in situ by the action of mineral acid on sodium nitrite
Sodium nitrite
Sodium nitrite is the inorganic compound with the chemical formula NaNO2. It is a white to slight yellowish crystalline powder that is very soluble in water and is hygroscopic...
:
- NaNO2 + HCl → HNO2 + NaCl
- 2 NaN3 + 2 HNO2 → 3 N2 + 2 NO + 2 NaOH
Reaction with two α-hydrogen atoms in ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s creates oxime
Oxime
An oxime is a chemical compound belonging to the imines, with the general formula R1R2C=NOH, where R1 is an organic side chain and R2 may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds...
s, which may be further oxidized to a carboxylic acid, or reduced to form amines. This process is used in the commercial production of adipic acid
Adipic acid
Adipic acid is the organic compound with the formula 42. From the industrial perspective, it is the most important dicarboxylic acid: About 2.5 billion kilograms of this white crystalline powder are produced annually, mainly as a precursor for the production of nylon...
.
Nitrous acid reacts rapidly with aliphatic alcohols
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
to produce alkyl nitrites
Alkyl nitrites
Alkyl nitrites are a group of chemical compounds based upon the molecular structure R-ONO. Formally they are alkyl esters of nitrous acid. They are distinct from nitro compounds ....
, which are potent vasodilators
Vasodilation
Vasodilation refers to the widening of blood vessels resulting from relaxation of smooth muscle cells within the vessel walls, particularly in the large arteries, smaller arterioles and large veins. The process is essentially the opposite of vasoconstriction, or the narrowing of blood vessels. When...
:
2CH-CH2-CH2-OH + HNO2 → (CH3)2CH-CH2-CH2-ONO + H2O
Atmosphere of the earth
Nitrous acid is involved in the ozoneOzone
Ozone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
budget of the lower atmosphere
Atmosphere
An atmosphere is a layer of gases that may surround a material body of sufficient mass, and that is held in place by the gravity of the body. An atmosphere may be retained for a longer duration, if the gravity is high and the atmosphere's temperature is low...
: the troposphere
Troposphere
The troposphere is the lowest portion of Earth's atmosphere. It contains approximately 80% of the atmosphere's mass and 99% of its water vapor and aerosols....
. The heterogeneous reaction of nitrogen monoxide (NO) and water produces nitrous acid. When this reaction takes place on the surface of atmospheric aerosol
Aerosol
Technically, an aerosol is a suspension of fine solid particles or liquid droplets in a gas. Examples are clouds, and air pollution such as smog and smoke. In general conversation, aerosol usually refers to an aerosol spray can or the output of such a can...
s, product readily photolyses to hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
radical
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
s.
See also
- Demjanov rearrangementDemjanov rearrangementThe Demjanov rearrangement is the chemical reaction of primary amines with nitrous acid to give rearranged alcohols. It involves substitution by a hydroxyl group with a possible ring expansion. It is named after the Russian chemist Nikolai Jakovlevich Demjanov...
- Nitric acidNitric acidNitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
(HHydrogenHydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
NNitrogenNitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
OOxygenOxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
3) - Tiffeneau-Demjanov rearrangementTiffeneau-Demjanov rearrangementThe Tiffeneau-Demjanov rearrangement is the chemical reaction of a 1-aminomethyl-cycloalkanol with nitrous acid to form an enlarged cycloketone....

