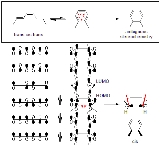
Disrotatory
Encyclopedia
In a conrotatory mode of an electrocyclic reaction
(a class of organic
chemical reaction
s) the substituent
s located at the termini of a conjugated
double bond
system move in the same (clockwise or counter clockwise) direction during ring opening or ring closure. In a disrotatory mode, they move in opposite directions.
A simple arrow-pushing analysis fails to explain the quantitative conversion of trans
-cis-trans-2,4,6-octatriene to cis
-dimethylcyclohexadiene (top of figure). However, a detailed look at the orbital mechanics behind the process reveals that the orbital symmetry of the octatriene's HOMO requires for the end pi orbitals to move in opposite directions to form the correct symmetry found in the sigma bond.
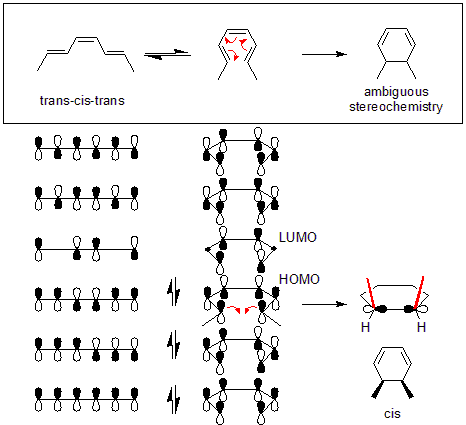 This stereospecificity is found in thermal rearrangements of all conjugated systems containing 4n + 2 pi electron and is based on preservation of orbital symmetry in the highest occupied molecular orbital
This stereospecificity is found in thermal rearrangements of all conjugated systems containing 4n + 2 pi electron and is based on preservation of orbital symmetry in the highest occupied molecular orbital
. Systems containing 4n pi electrons show the opposite conrotatory mode, as do photoinduced rearrangements of 4n + 2 pi electrons. Photoinduced rearrangements of 4n electron systems follow the disrotatory rule.
The Woodward-Hoffmann rules
summarize the above different reactions.
Electrocyclic reaction
In organic chemistry, an electrocyclic reaction is a type of pericyclic rearrangement reaction where the net result is one pi bond being converted into one sigma bond or vice-versa...
(a class of organic
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
s) the substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
s located at the termini of a conjugated
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
double bond
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
system move in the same (clockwise or counter clockwise) direction during ring opening or ring closure. In a disrotatory mode, they move in opposite directions.
A simple arrow-pushing analysis fails to explain the quantitative conversion of trans
Geometric isomerism
In organic chemistry, cis/trans isomerism or geometric isomerism or configuration isomerism or E/Z isomerism is a form of stereoisomerism describing the orientation of functional groups within a molecule...
-cis-trans-2,4,6-octatriene to cis
Geometric isomerism
In organic chemistry, cis/trans isomerism or geometric isomerism or configuration isomerism or E/Z isomerism is a form of stereoisomerism describing the orientation of functional groups within a molecule...
-dimethylcyclohexadiene (top of figure). However, a detailed look at the orbital mechanics behind the process reveals that the orbital symmetry of the octatriene's HOMO requires for the end pi orbitals to move in opposite directions to form the correct symmetry found in the sigma bond.

Homo
Homo may refer to:*the Greek prefix ὅμο-, meaning "the same"*the Latin for man, human being*Homo, the taxonomical genus including modern humans...
. Systems containing 4n pi electrons show the opposite conrotatory mode, as do photoinduced rearrangements of 4n + 2 pi electrons. Photoinduced rearrangements of 4n electron systems follow the disrotatory rule.
The Woodward-Hoffmann rules
Woodward-Hoffmann rules
The Woodward–Hoffmann rules devised by Robert Burns Woodward and Roald Hoffmann are a set of rules in organic chemistry predicting the stereochemistry of pericyclic reactions based on orbital symmetry. These include electrocyclic reactions, cycloadditions , sigmatropic reactions, and group transfer...
summarize the above different reactions.

