
Helium-4
Encyclopedia

Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
of helium
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
. It is by far the most abundant of the two naturally occurring isotopes of helium, making up about 99.99986% of the helium on earth. Its nucleus is the same as an alpha particle
Alpha particle
Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus, which is classically produced in the process of alpha decay, but may be produced also in other ways and given the same name...
, consisting of two proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
s and two neutron
Neutron
The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
s. Alpha decay
Alpha decay
Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle and thereby transforms into an atom with a mass number 4 less and atomic number 2 less...
of heavy elements in the Earth's crust is the source of most naturally occurring helium-4 on Earth. Helium-4 is also produced by nuclear fusion
Nuclear fusion
Nuclear fusion is the process by which two or more atomic nuclei join together, or "fuse", to form a single heavier nucleus. This is usually accompanied by the release or absorption of large quantities of energy...
in star
Star
A star is a massive, luminous sphere of plasma held together by gravity. At the end of its lifetime, a star can also contain a proportion of degenerate matter. The nearest star to Earth is the Sun, which is the source of most of the energy on Earth...
s. Most of the helium-4 in the universe, however (including most of the helium in the Sun), was thought to have been produced by the Big Bang. Helium-4 makes up about a quarter of the ordinary matter in the universe, with almost all of the rest being hydrogen. However, this primordial helium is largely absent from the Earth, having escaped during the high temperature phase of Earth's formation, leaving radioactive decay to produce most helium on Earth, after the planet cooled and solidified.
When helium-4 is cooled to below 2.17 kelvins (–271 °C), it becomes a superfluid
Superfluid
Superfluidity is a state of matter in which the matter behaves like a fluid without viscosity and with extremely high thermal conductivity. The substance, which appears to be a normal liquid, will flow without friction past any surface, which allows it to continue to circulate over obstructions and...
, with properties that are very unlike those of an ordinary liquid. For example, if helium-4 is kept in an open vessel, a thin film will climb up the sides of the vessel and overflow. Another name for this property of helium is Rollin film
Rollin film
A Rollin film, named after Bernard V. Rollin, is a 30 nm-thick liquid film of helium in the helium II state. It exhibits a "creeping" effect in response to surfaces extending past the film's level...
. This strange behaviour is a result of the Clausius-Clapeyron relation
Clausius-Clapeyron relation
The Clausius–Clapeyron relation, named after Rudolf Clausius and Benoît Paul Émile Clapeyron, who defined it sometime after 1834, is a way of characterizing a discontinuous phase transition between two phases of matter. On a pressure–temperature diagram, the line separating the two phases is known...
and cannot be explained by the current model
Model (physical)
A physical model is a smaller or larger physical copy of an object...
of classical mechanics
Classical mechanics
In physics, classical mechanics is one of the two major sub-fields of mechanics, which is concerned with the set of physical laws describing the motion of bodies under the action of a system of forces...
nor by nuclear
Nuclear physics
Nuclear physics is the field of physics that studies the building blocks and interactions of atomic nuclei. The most commonly known applications of nuclear physics are nuclear power generation and nuclear weapons technology, but the research has provided application in many fields, including those...
or electrical models; it is only understood as a quantum mechanical
Quantum mechanics
Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
phenomenon. The total spin of the nucleus (zero) is an integer, so it is a boson
Boson
In particle physics, bosons are subatomic particles that obey Bose–Einstein statistics. Several bosons can occupy the same quantum state. The word boson derives from the name of Satyendra Nath Bose....
, as are neutral atoms of helium-4. The superfluid behavior is now understood to be a manifestation of Bose-Einstein condensation, which occurs only with bosons.
Helium-4 also exists on the moon, and as with on Earth, is the most abundant helium isotope.
The helium-4 atom
The helium atom is the next least complicated atom after hydrogen, but the extra electron introduces a third body, so that solution becomes a three body problem which has no analytic solution. However, numerical approximations of the equations of quantum mechanics have given a good estimate of the key atomic properties (such as size and ionization energy) of helium-4.The related stability of the helium-4 nucleus and electron shell
The nucleus of the helium-4 atom is identical with an alpha particleAlpha particle
Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus, which is classically produced in the process of alpha decay, but may be produced also in other ways and given the same name...
. High energy electron-scattering experiments show its charge to decrease exponentially from a maximum at a central point, exactly as does the charge density of helium's own electron cloud. This symmetry reflects similar underlying physics: the pair of neutrons and the pair of protons in helium's nucleus obey the same quantum mechanical rules as do helium's pair of electrons (although the nuclear particles are subject to a different nuclear binding potential), so that all these fermion
Fermion
In particle physics, a fermion is any particle which obeys the Fermi–Dirac statistics . Fermions contrast with bosons which obey Bose–Einstein statistics....
s fully occupy 1s1s orbitals in pairs, none of them possessing orbital angular momentum, and each cancelling the other's intrinsic spin. Adding another of any of these particles would require angular momentum and would release substantially less energy (in fact, no nucleus with five nucleons is stable). This arrangement is thus energetically extremely stable for all these particles, and this stability accounts for many crucial facts regarding helium in nature.
For example, the stability and low energy of the electron cloud state in helium accounts for the element's chemical inertness (the most extreme of all the elements), and also the lack of interaction of helium atoms with each other, producing the lowest melting and boiling points of all the elements.
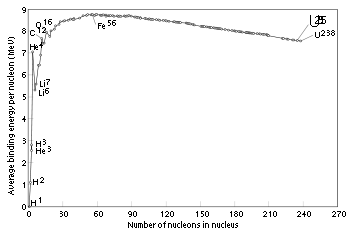
In a similar way, the particular energetic stability of the helium-4 nucleus, produced by similar effects, accounts for the ease of helium-4 production in atomic reactions involving both heavy-particle emission, and fusion. Some stable helium-3 is produced in fusion reactions from hydrogen, but it is a very small fraction, compared with the highly favorable helium-4. The stability of helium-4 is the reason hydrogen is converted to helium-4 (not deuterium or helium-3 or heavier elements) in the Sun. It is also partly responsible for the fact that the alpha particle is by far the most common type of baryonic particle to be ejected from atomic nuclei; in other words, alpha decay
Alpha decay
Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle and thereby transforms into an atom with a mass number 4 less and atomic number 2 less...
is far more common than cluster decay
Cluster decay
Cluster decay is a type of nuclear decay in which a parent atomic nucleus with A nucleons and Z protons emits a cluster of Ne neutrons and Ze protons heavier than an alpha particle but lighter than a typical binary fission fragment Cluster decay (also named heavy particle radioactivity or heavy...
.
Big Bang
The Big Bang theory is the prevailing cosmological model that explains the early development of the Universe. According to the Big Bang theory, the Universe was once in an extremely hot and dense state which expanded rapidly. This rapid expansion caused the young Universe to cool and resulted in...
, as the "soup" of free protons and neutrons which had initially been created in about 6:1 ratio cooled to the point that nuclear binding was possible, almost all first compound atomic nuclei to form were helium-4 nuclei. So tight was helium-4 binding that helium-4 production consumed nearly all of the free neutrons in a few minutes, before they could beta-decay, and also leaving few to form heavier atoms such as lithium, beryllium, or boron. Helium-4 nuclear binding per nucleon is stronger than in any of these elements (see nucleogenesis and binding energy
Binding energy
Binding energy is the mechanical energy required to disassemble a whole into separate parts. A bound system typically has a lower potential energy than its constituent parts; this is what keeps the system together—often this means that energy is released upon the creation of a bound state...
) and thus no energetic drive was available, once helium had been formed, to make elements 3, 4 and 5. It was barely energetically favorable for helium to fuse into the next element with a lower energy per nucleon
Nucleon
In physics, a nucleon is a collective name for two particles: the neutron and the proton. These are the two constituents of the atomic nucleus. Until the 1960s, the nucleons were thought to be elementary particles...
, carbon. However, due to lack of intermediate elements, this process requires three helium nuclei striking each other nearly simultaneously (see triple alpha process). There was thus no time for significant carbon to be formed in the few minutes after the Big Bang, before the early expanding universe cooled to the temperature and pressure point where helium fusion to carbon was no longer possible. This left the early universe with a very similar ratio of hydrogen/helium as is observed today (3 parts hydrogen to 1 part helium-4 by mass), with nearly all the neutrons in the universe trapped in helium-4.
All heavier elements (including those necessary for rocky planets like the Earth, and for carbon-based or other life), have thus had to be created since the Big Bang, in stars which were hot enough to fuse not just hydrogen (for this produces only more helium), but to fuse helium itself. All elements other than hydrogen and helium today account for only 2% of the mass of atomic matter in the universe. Helium-4, by contrast, makes up about 23% of the universe's ordinary matter—nearly all the ordinary matter that isn't hydrogen.

