Nucleophile
Encyclopedia
A nucleophile is a species
that donates an electron-pair to an electrophile
to form a chemical bond
in a reaction
. All molecule
s or ion
s with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.
Nucleophilic describes the affinity of a nucleophile to the nuclei. Nucleophilicity, sometimes referred to as nucleophile strength, refers to a substance's nucleophilic character and is often used to compare the affinity of atoms.
Neutral nucleophilic reactions with solvents such as alcohol
s and water are named solvolysis
. Nucleophiles may take part in nucleophilic substitution
, whereby a nucleophile becomes attracted to a full or partial positive charge.
in 1929, replacing the terms cationoid and anionoid proposed earlier by A. J. Lapworth in 1925.
The word nucleophile is derived from nucleus
and the Greek word φιλος, philos
for love.
is more important in the determination of the nucleophilicity: The easier it is to distort the electron cloud around an atom or molecule the more readily it will react; e.g., the iodide
ion (I−) is more nucleophilic than the fluoride
ion (F−).
data have been obtained by measuring reaction rate
s for a large number of reactions involving a large number of nucleophiles and electrophiles. Nucleophiles displaying the so-called alpha effect
are usually omitted in this type of treatment.

This free-energy relationship
relates the pseudo first order reaction rate constant (in water at 25 °C), k, of a reaction, normalized to the reaction rate, k0, of a standard reaction with water as the nucleophile, to a nucleophilic constant n for a given nucleophile and a substrate constant s that depends on the sensitivity of a substrate to nucleophilic attack (defined as 1 for methyl bromide).
This treatment results in the following values for typical nucleophilic anions: acetate
2.7, chloride
3.0, azide
4.0, hydroxide
4.2, aniline
4.5, iodide
5.0, and thiosulfate
6.4. Typical substrate constants are 0.66 for ethyl tosylate, 0.77 for β-propiolactone
, 1.00 for 2,3-epoxypropanol
, 0.87 for benzyl chloride
, and 1.43 for benzoyl chloride
.
The equation predicts that, in a nucleophilic displacement on benzyl chloride
, the azide
anion reacts 3000 times faster than water.
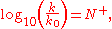
where N+ is the nucleophile dependent parameter and k0 the reaction rate constant for water. In this equation, a substrate-dependent parameter like s in the Swain–Scott equation is absent. The equation states that two nucleophiles react with the same relative reactivity regardless of the nature of the electrophile, which is in violation of the Reactivity–selectivity principle
. For this reason this equation is also called the constant selectivity relationship.
In the original publication the data were obtained by reactions of selected nucleophiles with selected electrophilic carbocation
s such as tropylium cations:
or diazonium cations:
or (not displayed) ions based on Malachite green
. Many other reaction types have since been described.
Typical Ritchie N+ values (in methanol
) are: 0.5 for methanol
, 5.9 for the cyanide
anion, 7.5 for the methoxide
anion, 8.5 for the azide
anion, and 10.7 for the thiophenol
anion. The values for the relative cation reactivities are -0.4 for the malachite green cation, +2.6 for the benzenediazonium cation, and +4.5 for the tropylium cation.
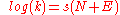
The second order reaction rate constant k at 20°C for a reaction is related to a nucleophilicity parameter N, an electrophilicity parameter E, and a nucleophile-dependent slope parameter s. The constant s is defined as 1 with 2-methyl-1-pentene as the nucleophile.
Many of the constants have been derived from reaction of so-called benzhydrylium ions as the electrophile
s:
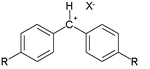 and a diverse collection of π-nucleophiles:
and a diverse collection of π-nucleophiles:
Typical E values are +6.2 for R = chlorine
, +5.90 for R = hydrogen
, 0 for R = methoxy
and -7.02 for R = dimethylamine
.
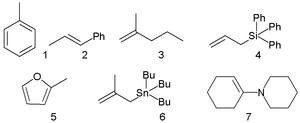
Typical N values with s in parenthesis are -4.47 (1.32) for electrophilic aromatic substitution
to toluene
(1), -0.41 (1.12) for electrophilic addition
to 1-phenyl-2-propene (2), and 0.96 (1) for addition to 2-methyl-1-pentene (3), -0.13 (1.21) for reaction with triphenylallylsilane (4), 3.61 (1.11) for reaction with 2-methylfuran
(5), +7.48 (0.89) for reaction with isobutenyltributylstannane (6) and +13.36 (0.81) for reaction with the enamine
7.
The range of organic reactions also include SN2 reaction
s:
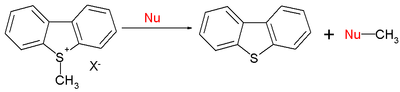 With E = -9.15 for the S-methyldibenzothiophenium ion, typical nucleophile values N (s) are 15.63 (0.64) for piperidine
With E = -9.15 for the S-methyldibenzothiophenium ion, typical nucleophile values N (s) are 15.63 (0.64) for piperidine
, 10.49 (0.68) for methoxide
, and 5.20 (0.89) for water. In short, nucleophilicities towards sp2 or sp3 centers follow the same pattern.

with sE the electrophile-dependent slope parameter and sN the nucleophile-dependent slope parameter. This equation can be rewritten in several ways:
In the example below, the oxygen
of the hydroxide ion donates an electron pair to bond with the carbon
at the end of the bromopropane molecule. The bond between the carbon and the bromine
then undergoes heterolytic fission, with the bromine atom taking the donated electron and becoming the bromide
ion (Br−), because a SN2 reaction occurs by backside attack. This means that the hydroxide ion attacks the carbon atom from the other side, exactly opposite the bromine ion. Because of this backside attack, SN2 reactions result in a reversal of the configuration
of the electrophile. If the electrophile is chiral, it typically maintains its chirality, though the SN2 product's configuration is flipped as compared to that of the original electrophile.

An ambident nucleophile is one that can attack from two or more places, resulting in two or more products. For example, the thiocyanate
ion (SCN−) may attack from either the or the . For this reason, the SN2 reaction
of an alkyl halide with SCN− often leads to a mixture of RSCN (an alkyl thiocyanate) and RNCS (an alkyl isothiocyanate
). Similar considerations apply in the Kolbe nitrile synthesis
.
, Blaise reaction
, Reformatsky reaction, and Barbier reaction
, organolithium reagent
s, and anions of a terminal
alkyne
.
Enols are also carbon nucleophiles. The formation of an enol is catalyzed by acid or base. Enols are ambident nucleophiles, but, in general, nucleophilic at the alpha carbon atom.
Enols are commonly used in condensation reactions, including the Claisen condensation and the aldol condensation reactions.
(H2O), hydroxide
anion, alcohol
s, alkoxide
anions, hydrogen peroxide
, and carboxylate anions.
and its salts, thiols
(RSH), thiolate anions (RS−), anions of thiolcarboxylic acids (RC(O)-S−), and anions of dithiocarbonates (RO-C(S)-S−) and dithiocarbamates (R2N-C(S)-S−) are used most often.
In general, sulfur is very nucleophilic because of its large size, which makes it readily polarizable, and its lone pairs of electrons are readily accessible.
, azide
, amine
s, and nitrites
.
Chemical species
Chemical species are atoms, molecules, molecular fragments, ions, etc., being subjected to a chemical process or to a measurement. Generally, a chemical species can be defined as an ensemble of chemically identical molecular entities that can explore the same set of molecular energy levels on a...
that donates an electron-pair to an electrophile
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
to form a chemical bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
in a reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
. All molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s or ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.
Nucleophilic describes the affinity of a nucleophile to the nuclei. Nucleophilicity, sometimes referred to as nucleophile strength, refers to a substance's nucleophilic character and is often used to compare the affinity of atoms.
Neutral nucleophilic reactions with solvents such as alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s and water are named solvolysis
Solvolysis
Solvolysis is a special type of nucleophilic substitution or elimination where the nucleophile is a solvent molecule. For certain nucleophiles, there are specific terms for the type of solvolysis reaction...
. Nucleophiles may take part in nucleophilic substitution
Nucleophilic substitution
In organic and inorganic chemistry, nucleophilic substitution is a fundamental class of reactions in which an electron nucleophile selectively bonds with or attacks the positive or partially positive charge of an atom or a group of atoms called the leaving group; the positive or partially positive...
, whereby a nucleophile becomes attracted to a full or partial positive charge.
History
The terms nucleophile and electrophile were introduced by Christopher Kelk IngoldChristopher Kelk Ingold
Sir Christopher Kelk Ingold FRS was a British chemist based in Leeds and London. His groundbreaking work in the 1920s and 1930s on reaction mechanisms and the electronic structure of organic compounds was responsible for the introduction into mainstream chemistry of concepts such as nucleophile,...
in 1929, replacing the terms cationoid and anionoid proposed earlier by A. J. Lapworth in 1925.
The word nucleophile is derived from nucleus
Atomic nucleus
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
and the Greek word φιλος, philos
-phil-
Suffixes with the common part -phil- are used to specify some kind of attraction or affinity to something, in particular the love or obsession with something...
for love.
Properties
In general, in a row across the periodic table, the more basic the ion (the higher the pKa of the conjugate acid) the more reactive it is as a nucleophile. In a given group, polarizabilityPolarizability
Polarizability is the measure of the change in a molecule's electron distribution in response to an applied electric field, which can also be induced by electric interactions with solvents or ionic reagents. It is a property of matter...
is more important in the determination of the nucleophilicity: The easier it is to distort the electron cloud around an atom or molecule the more readily it will react; e.g., the iodide
Iodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. This page is for the iodide ion and its salts. For information on organoiodides, see organohalides. In everyday life, iodide is most commonly encountered as a component of iodized salt,...
ion (I−) is more nucleophilic than the fluoride
Fluoride
Fluoride is the anion F−, the reduced form of fluorine when as an ion and when bonded to another element. Both organofluorine compounds and inorganic fluorine containing compounds are called fluorides. Fluoride, like other halides, is a monovalent ion . Its compounds often have properties that are...
ion (F−).
Nucleophilicity scales
Many schemes attempting to quantify relative nucleophilic strength have been devised. The following empiricalEmpirical
The word empirical denotes information gained by means of observation or experimentation. Empirical data are data produced by an experiment or observation....
data have been obtained by measuring reaction rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
s for a large number of reactions involving a large number of nucleophiles and electrophiles. Nucleophiles displaying the so-called alpha effect
Alpha Effect
The alpha effect refers to the increased nucleophilicity of a molecule due to the presence of an adjacent atom with lone pair electrons. The molecule does not necessarily exhibit increased basicity compared with a similar molecule without the adjacent, electron donating atom...
are usually omitted in this type of treatment.
Swain-Scott equation
The first such attempt is found in the Swain–Scott equation derived in 1953:
This free-energy relationship
Free-energy relationship
In physical organic chemistry, a free-energy relationship or linear Gibbs energy relation relates the logarithm of a reaction rate constant or equilibrium constant for one series of reactions with the logarithm of the rate or equilibrium constant for a related series of reactions...
relates the pseudo first order reaction rate constant (in water at 25 °C), k, of a reaction, normalized to the reaction rate, k0, of a standard reaction with water as the nucleophile, to a nucleophilic constant n for a given nucleophile and a substrate constant s that depends on the sensitivity of a substrate to nucleophilic attack (defined as 1 for methyl bromide).
This treatment results in the following values for typical nucleophilic anions: acetate
Acetate
An acetate is a derivative of acetic acid. This term includes salts and esters, as well as the anion found in solution. Most of the approximately 5 billion kilograms of acetic acid produced annually in industry are used in the production of acetates, which usually take the form of polymers. In...
2.7, chloride
Chloride
The chloride ion is formed when the element chlorine, a halogen, picks up one electron to form an anion Cl−. The salts of hydrochloric acid HCl contain chloride ions and can also be called chlorides. The chloride ion, and its salts such as sodium chloride, are very soluble in water...
3.0, azide
Azide
Azide is the anion with the formula N3−. It is the conjugate base of hydrazoic acid. N3− is a linear anion that is isoelectronic with CO2 and N2O. Per valence bond theory, azide can be described by several resonance structures, an important one being N−=N+=N−...
4.0, hydroxide
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
4.2, aniline
Aniline
Aniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Being a precursor to many industrial chemicals, its main use is in the manufacture of precursors to polyurethane...
4.5, iodide
Iodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. This page is for the iodide ion and its salts. For information on organoiodides, see organohalides. In everyday life, iodide is most commonly encountered as a component of iodized salt,...
5.0, and thiosulfate
Thiosulfate
Thiosulfate is an oxyanion of sulfur. The prefix thio indicates that thiosulfate ion is a sulfate ion with one oxygen replaced by a sulfur. Thiosulfate occurs naturally and is produced by certain biochemical processes...
6.4. Typical substrate constants are 0.66 for ethyl tosylate, 0.77 for β-propiolactone
Lactone
In chemistry, a lactone is a cyclic ester which can be seen as the condensation product of an alcohol group -OH and a carboxylic acid group -COOH in the same molecule...
, 1.00 for 2,3-epoxypropanol
Epoxide
An epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in...
, 0.87 for benzyl chloride
Benzyl chloride
Benzyl chloride, or α-chlorotoluene, is an organic compound with the formula C6H5CH2Cl. This colourless liquid is a reactive organochlorine compound that is a widely used chemical building block.-Preparation:...
, and 1.43 for benzoyl chloride
Benzoyl chloride
Benzoyl chloride, also known as benzenecarbonyl chloride, is an organochlorine compound with the formula C6H5COCl. It is a colourless, fuming liquid with an irritating odour...
.
The equation predicts that, in a nucleophilic displacement on benzyl chloride
Benzyl chloride
Benzyl chloride, or α-chlorotoluene, is an organic compound with the formula C6H5CH2Cl. This colourless liquid is a reactive organochlorine compound that is a widely used chemical building block.-Preparation:...
, the azide
Azide
Azide is the anion with the formula N3−. It is the conjugate base of hydrazoic acid. N3− is a linear anion that is isoelectronic with CO2 and N2O. Per valence bond theory, azide can be described by several resonance structures, an important one being N−=N+=N−...
anion reacts 3000 times faster than water.
Ritchie equation
The Ritchie equation, derived in 1972, is another free-energy relationship: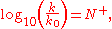
where N+ is the nucleophile dependent parameter and k0 the reaction rate constant for water. In this equation, a substrate-dependent parameter like s in the Swain–Scott equation is absent. The equation states that two nucleophiles react with the same relative reactivity regardless of the nature of the electrophile, which is in violation of the Reactivity–selectivity principle
Reactivity–selectivity principle
In chemistry the reactivity–selectivity principle or RSP states that a more reactive chemical compound or reactive intermediate is less selective in chemical reactions. In this context selectivity represents the ratio of reaction rates....
. For this reason this equation is also called the constant selectivity relationship.
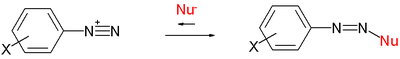
In the original publication the data were obtained by reactions of selected nucleophiles with selected electrophilic carbocation
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
s such as tropylium cations:
or diazonium cations:
or (not displayed) ions based on Malachite green
Malachite green
Malachite green is an organic compound that is used as a dyestuff and has emerged as a controversial agent in aquaculture. Malachite green is traditionally used as a dye for materials such as silk, leather, and paper...
. Many other reaction types have since been described.
Typical Ritchie N+ values (in methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
) are: 0.5 for methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
, 5.9 for the cyanide
Cyanide
A cyanide is a chemical compound that contains the cyano group, -C≡N, which consists of a carbon atom triple-bonded to a nitrogen atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic....
anion, 7.5 for the methoxide
Methoxide
Methoxides are organic salts and the simplest alkoxides. Sodium methoxide and potassium hydroxide have widespread use, though other variants such as lithium hydroxide, rubidium methoxide, caesium methoxide, and francium methoxide exist as well.- Methoxide ion :In organic chemistry, the methoxide...
anion, 8.5 for the azide
Azide
Azide is the anion with the formula N3−. It is the conjugate base of hydrazoic acid. N3− is a linear anion that is isoelectronic with CO2 and N2O. Per valence bond theory, azide can be described by several resonance structures, an important one being N−=N+=N−...
anion, and 10.7 for the thiophenol
Thiophenol
Thiophenol is an organosulfur compound with the formula C6H6S, and sometimes abbreviated as PhSH. This foul-smelling colourless liquid is the simplest aromatic thiol. The chemical structures of thiophenols are analogous to phenols except the oxygen atom in the hydroxyl group bonded to the...
anion. The values for the relative cation reactivities are -0.4 for the malachite green cation, +2.6 for the benzenediazonium cation, and +4.5 for the tropylium cation.
Mayr-Patz equation
In the Mayr-Patz equation (1994):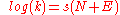
The second order reaction rate constant k at 20°C for a reaction is related to a nucleophilicity parameter N, an electrophilicity parameter E, and a nucleophile-dependent slope parameter s. The constant s is defined as 1 with 2-methyl-1-pentene as the nucleophile.
Many of the constants have been derived from reaction of so-called benzhydrylium ions as the electrophile
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
s:

- .
Typical E values are +6.2 for R = chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
, +5.90 for R = hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
, 0 for R = methoxy
Methoxy
In chemistry , methoxy refers to the functional group consisting of a methyl group bound to oxygen. This alkoxy group has the formula O–CH3.The word is used in organic nomenclature usually to describe an ether...
and -7.02 for R = dimethylamine
Dimethylamine
Dimethylamine is an organic compound with the formula 2NH. This secondary amine is a colorless, flammable liquified gas with an ammonia-like odor. Dimethylamine is generally encountered as a solution in water at concentrations up to around 40%...
.
Typical N values with s in parenthesis are -4.47 (1.32) for electrophilic aromatic substitution
Electrophilic aromatic substitution
Electrophilic aromatic substitution EAS is an organic reaction in which an atom, usually hydrogen, appended to an aromatic system is replaced by an electrophile...
to toluene
Toluene
Toluene, formerly known as toluol, is a clear, water-insoluble liquid with the typical smell of paint thinners. It is a mono-substituted benzene derivative, i.e., one in which a single hydrogen atom from the benzene molecule has been replaced by a univalent group, in this case CH3.It is an aromatic...
(1), -0.41 (1.12) for electrophilic addition
Electrophilic addition
In organic chemistry, an electrophilic addition reaction is an addition reaction where, in a chemical compound, a π bond is broken and two new σ bonds are formed...
to 1-phenyl-2-propene (2), and 0.96 (1) for addition to 2-methyl-1-pentene (3), -0.13 (1.21) for reaction with triphenylallylsilane (4), 3.61 (1.11) for reaction with 2-methylfuran
2-Methylfuran
2-Methylfuran is a flammable, water-soluble liquid with a chocolate odor, found naturally in Myrtle and Dutch Lavenderused as a FEMA GRAS flavoring substance, with the potential for use in alternative fuels.-External links:*...
(5), +7.48 (0.89) for reaction with isobutenyltributylstannane (6) and +13.36 (0.81) for reaction with the enamine
Enamine
An enamine is an unsaturated compound derived by the reaction of an aldehyde or ketone with a secondary amine followed by loss of H2O.The word "enamine" is derived from the affix en-, used as the suffix of alkene, and the root amine. This can be compared with enol, which is a functional group...
7.
The range of organic reactions also include SN2 reaction
SN2 reaction
The SN2 reaction is a type of nucleophilic substitution, where a lone pair from a nucleophile attacks an electron deficient electrophilic center and bonds to it, expelling another group called a leaving group. Thus the incoming group replaces the leaving group in one step...
s:

Piperidine
Piperidine is an organic compound with the molecular formula 5NH. This heterocyclic amine consists of a six-membered ring containing five methylene units and one nitrogen atom...
, 10.49 (0.68) for methoxide
Methoxide
Methoxides are organic salts and the simplest alkoxides. Sodium methoxide and potassium hydroxide have widespread use, though other variants such as lithium hydroxide, rubidium methoxide, caesium methoxide, and francium methoxide exist as well.- Methoxide ion :In organic chemistry, the methoxide...
, and 5.20 (0.89) for water. In short, nucleophilicities towards sp2 or sp3 centers follow the same pattern.
Unified equation
In an effort to unify the above described equations the Mayr equation is rewritten as:
with sE the electrophile-dependent slope parameter and sN the nucleophile-dependent slope parameter. This equation can be rewritten in several ways:
- with sE = 1 for carbocations this equation is equal to the original Mayr-Patz equation of 1994,
- with sN = 0.6 for most n nucleophiles the equation becomes
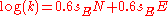
- or the original Scott-Swain equation written as:
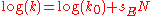
- with sE = 1 for carbocations and sN = 0.6 the equation becomes:
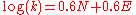
- with sE = 1 for carbocations and sN = 0.6 the equation becomes:
- or the original Ritchie equation written as:
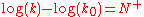
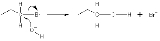
Types of nucleophiles
Examples of nucleophiles are anions such as Cl−, or a compound with a lone pair of electrons such as NH3 (ammonia).In the example below, the oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
of the hydroxide ion donates an electron pair to bond with the carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
at the end of the bromopropane molecule. The bond between the carbon and the bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
then undergoes heterolytic fission, with the bromine atom taking the donated electron and becoming the bromide
Bromide
A bromide is a chemical compound containing bromide ion, that is bromine atom with effective charge of −1. The class name can include ionic compounds such as caesium bromide or covalent compounds such as sulfur dibromide.-Natural occurrence:...
ion (Br−), because a SN2 reaction occurs by backside attack. This means that the hydroxide ion attacks the carbon atom from the other side, exactly opposite the bromine ion. Because of this backside attack, SN2 reactions result in a reversal of the configuration
Molecular configuration
The configuration of a molecule is the permanent geometry that results from the spatial arrangement of its bonds. The ability of the same set of atoms to form two or more molecules with different configurations is stereoisomerism...
of the electrophile. If the electrophile is chiral, it typically maintains its chirality, though the SN2 product's configuration is flipped as compared to that of the original electrophile.

An ambident nucleophile is one that can attack from two or more places, resulting in two or more products. For example, the thiocyanate
Thiocyanate
Thiocyanate is the anion [SCN]−. It is the conjugate base of thiocyanic acid. Common derivatives include the colourless salts potassium thiocyanate and sodium thiocyanate. Organic compounds containing the functional group SCN are also called thiocyanates...
ion (SCN−) may attack from either the or the . For this reason, the SN2 reaction
SN2 reaction
The SN2 reaction is a type of nucleophilic substitution, where a lone pair from a nucleophile attacks an electron deficient electrophilic center and bonds to it, expelling another group called a leaving group. Thus the incoming group replaces the leaving group in one step...
of an alkyl halide with SCN− often leads to a mixture of RSCN (an alkyl thiocyanate) and RNCS (an alkyl isothiocyanate
Isothiocyanate
Isothiocyanate is the chemical group –N=C=S, formed by substituting sulfur for oxygen in the isocyanate group. Many natural isothiocyanates from plants are produced by enzymatic conversion of metabolites called glucosinolates. These natural isothiocyanates, such as allyl isothiocyanate, are also...
). Similar considerations apply in the Kolbe nitrile synthesis
Kolbe nitrile synthesis
The Kolbe nitrile synthesis is a method for the preparation of alkyl nitriles by reaction of the corresponding alkylhalide with a metal cyanide . A side product for this reaction is the formation of an isonitrile because the cyanide ion is an ambident nucleophile and according to Kornblum's rule is...
.
Carbon nucleophiles
Carbon nucleophiles are alkyl metal halides found in the Grignard reactionGrignard reaction
The Grignard reaction is an organometallic chemical reaction in which alkyl- or aryl-magnesium halides add to a carbonyl group in an aldehyde or ketone. This reaction is an important tool for the formation of carbon–carbon bonds...
, Blaise reaction
Blaise reaction
The Blaise reaction is an organic reaction that forms a β-ketoester from the reaction of zinc metal with a α-bromoester and a nitrile. The final intermediate is a metaloimine, which is hydrolyzed to give the desired β-ketoester....
, Reformatsky reaction, and Barbier reaction
Barbier reaction
The Barbier reaction is an organic reaction between an alkyl halide and a carbonyl group as an electrophilic substrate in the presence of magnesium, aluminium, zinc, indium, tin or its salts. The reaction product is a primary, secondary or tertiary alcohol...
, organolithium reagent
Organolithium reagent
An organolithium reagent is an organometallic compound with a direct bond between a carbon and a lithium atom. As the electropositive nature of lithium puts most of the charge density of the bond on the carbon atom, effectively creating a carbanion, organolithium compounds are extremely powerful...
s, and anions of a terminal
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
.
Enols are also carbon nucleophiles. The formation of an enol is catalyzed by acid or base. Enols are ambident nucleophiles, but, in general, nucleophilic at the alpha carbon atom.
Enols are commonly used in condensation reactions, including the Claisen condensation and the aldol condensation reactions.
Oxygen nucleophiles
Examples of oxygen nucleophiles are waterWater
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
(H2O), hydroxide
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
anion, alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s, alkoxide
Alkoxide
An alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They can be written as RO−, where R is the organic substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands...
anions, hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
, and carboxylate anions.
Sulfur nucleophiles
Of sulfur nucleophiles, hydrogen sulfideHydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
and its salts, thiols
Thiol
In organic chemistry, a thiol is an organosulfur compound that contains a carbon-bonded sulfhydryl group...
(RSH), thiolate anions (RS−), anions of thiolcarboxylic acids (RC(O)-S−), and anions of dithiocarbonates (RO-C(S)-S−) and dithiocarbamates (R2N-C(S)-S−) are used most often.
In general, sulfur is very nucleophilic because of its large size, which makes it readily polarizable, and its lone pairs of electrons are readily accessible.
Nitrogen nucleophiles
Nitrogen nucleophiles include ammoniaAmmonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
, azide
Azide
Azide is the anion with the formula N3−. It is the conjugate base of hydrazoic acid. N3− is a linear anion that is isoelectronic with CO2 and N2O. Per valence bond theory, azide can be described by several resonance structures, an important one being N−=N+=N−...
, amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s, and nitrites
Nitrite
The nitrite ion has the chemical formula NO2−. The anion is symmetric with equal N-O bond lengths and a O-N-O bond angle of ca. 120°. On protonation the unstable weak acid nitrous acid is produced. Nitrite can be oxidised or reduced, with product somewhat dependent on the oxidizing/reducing agent...
.