Epoxide
Encyclopedia
An epoxide is a cyclic ether
with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained
. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide
or oxirane, such as in chloromethyloxirane. As a functional group
, epoxides feature the epoxy prefix, such as in the compound 1,2-epoxycycloheptane, which can also be called cycloheptene epoxide, or simply cycloheptene oxide.
 A polymer
A polymer
containing unreacted epoxide units is called a polyepoxide or an epoxy
. Epoxy resins are used as adhesive
s and structural materials. Polymerization of an epoxide gives a polyether, for example ethylene oxide
polymerizes to give polyethylene glycol
, also known as polyethylene oxide.
and propylene oxide
, which are produced respectively on the scales of approximately 15 and 3 million tonnes. The epoxidation of ethylene involves its catalytic reaction of oxygen according to the following stoichiometry
:
The direct reaction of oxygen with alkenes is useful only for this epoxide. Other alkenes fail to react usefully, even propylene
.
-containing reagents, which donate a single oxygen atom. Typical peroxide reagents include hydrogen peroxide, peroxycarboxylic acids (generated in-situ or preformed), and alkyl hydroperoxides. In specialized applications, other peroxide-containing reagents are employed, such as dimethyldioxirane
.
The largest scale application of this approach is the production of propylene oxide from propylene using either t-butyl hydroperoxide or ethylbenzene
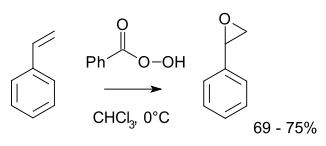
hydroperoxide. More typically for laboratory operations, the Prilezhaev reaction is employed. This approach involves the oxidation of the alkene with a peroxyacid such as m-CPBA
. Illustrative is the epoxidation of styrene with perbenzoic acid to styrene oxide
:
The reaction proceeds via what is commonly known as the "Butterfly Mechanism." The peroxide is viewed as an electrophile
, and the alkene a nucleophile
. The reaction is considered to be concerted (the numbers in the mechanism below are for simplification).
Hydroperoxides are also employed in catalytic enantioselective epoxidations, such as the Sharpless epoxidation
and the Jacobsen epoxidation
. In such cases, the oxygen is delivered from a metal oxide or peroxide. Together with the Shi epoxidation
, these reactions are useful for the enantioselective synthesis of chiral epoxides. Oxaziridine
reagents may also be used to generate epoxides from alkenes.
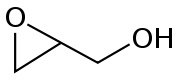
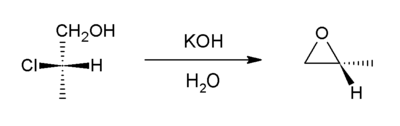
. In this case, an alkoxide ion displaces a chloride atom within the same molecule. The precursor compounds are called halohydrin
s. For example, with 2-chloropropanol:
Approximately, half of the world's supply of propylene oxide
arises via this route. An intramolecular epoxide formation reaction is one of the key steps in the Darzens reaction
.
In the Johnson-Corey-Chaykovsky reaction
epoxides are generated from carbonyl
groups and sulfonium ylides. In this reaction, a sulfonium is the leaving group instead of chloride.
s and acryl derivatives
can be epoxidized using nucleophilic oxygen compounds such as peroxides. The reaction is a two-step mechanism. First the oxygen performs a nucleophilic conjugate addition
to give a stabilized carbanion. This carbanion then attacks the same oxygen atom, displacing a leaving group from it, to close the epoxide ring.
, and thus may be stereogenic positions. Depending on the mechanism of the reaction and the geometry of the alkene starting material, cis and/or trans epoxide diastereomer
s may be formed. In addition, if there are other stereocenters present in the starting material, they can influence the stereochemistry of the epoxidation relative to them. This diastereoselectivity is a form of "substrate control" of the reaction. Finally, epoxidizing agents that possess stereogenic structures can influence the stereochemistry of the epoxide product (see for example the Sharpless epoxidation
, Jacobsen epoxidation
, and Juliá-Colonna Epoxidation
). This enantioselectivity is a form of "reagent control" of the reaction.
s. Perepoxides are proposed intermediates in the photosensitized oxidation of alkenes, as occurs when drying oil
s (a component of some paints and varnishes) are exposed to air in light. Such intermediates arise from the addition of singlet oxygen
to the double bond. Perepoxides rapidly rearrange to allylic hydroperoxides.
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained
Ring strain
In organic chemistry, ring strain is the tendency of a cyclic molecule, such as cyclopropane, to destabilize when its atoms are in non-favorable high energy spatial orientations...
. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide
Ethylene oxide
Ethylene oxide, also called oxirane, is the organic compound with the formula . It is a cyclic ether. This means that it is composed of two alkyl groups attached to an oxygen atom in a cyclic shape . This colorless flammable gas with a faintly sweet odor is the simplest epoxide, a three-membered...
or oxirane, such as in chloromethyloxirane. As a functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
, epoxides feature the epoxy prefix, such as in the compound 1,2-epoxycycloheptane, which can also be called cycloheptene epoxide, or simply cycloheptene oxide.

Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
containing unreacted epoxide units is called a polyepoxide or an epoxy
Epoxy
Epoxy, also known as polyepoxide, is a thermosetting polymer formed from reaction of an epoxide "resin" with polyamine "hardener". Epoxy has a wide range of applications, including fiber-reinforced plastic materials and general purpose adhesives....
. Epoxy resins are used as adhesive
Adhesive
An adhesive, or glue, is a mixture in a liquid or semi-liquid state that adheres or bonds items together. Adhesives may come from either natural or synthetic sources. The types of materials that can be bonded are vast but they are especially useful for bonding thin materials...
s and structural materials. Polymerization of an epoxide gives a polyether, for example ethylene oxide
Ethylene oxide
Ethylene oxide, also called oxirane, is the organic compound with the formula . It is a cyclic ether. This means that it is composed of two alkyl groups attached to an oxygen atom in a cyclic shape . This colorless flammable gas with a faintly sweet odor is the simplest epoxide, a three-membered...
polymerizes to give polyethylene glycol
Polyethylene glycol
Polyethylene glycol is a polyether compound with many applications from industrial manufacturing to medicine. It has also been known as polyethylene oxide or polyoxyethylene , depending on its molecular weight, and under the tradename Carbowax.-Available forms:PEG, PEO, or POE refers to an...
, also known as polyethylene oxide.
Synthesis
The dominant epoxides industrially are ethylene oxideEthylene oxide
Ethylene oxide, also called oxirane, is the organic compound with the formula . It is a cyclic ether. This means that it is composed of two alkyl groups attached to an oxygen atom in a cyclic shape . This colorless flammable gas with a faintly sweet odor is the simplest epoxide, a three-membered...
and propylene oxide
Propylene oxide
Propylene oxide is an organic compound with the molecular formula CH3CHCH2O. This colourless volatile liquid is produced on a large scale industrially, its major application being its use for the production of polyether polyols for use in making polyurethane plastics...
, which are produced respectively on the scales of approximately 15 and 3 million tonnes. The epoxidation of ethylene involves its catalytic reaction of oxygen according to the following stoichiometry
Stoichiometry
Stoichiometry is a branch of chemistry that deals with the relative quantities of reactants and products in chemical reactions. In a balanced chemical reaction, the relations among quantities of reactants and products typically form a ratio of whole numbers...
:
- 7 H2C=CH2 + 6 O2 → 6 C2H4O + 2 CO2 + 2 H2O
The direct reaction of oxygen with alkenes is useful only for this epoxide. Other alkenes fail to react usefully, even propylene
Propylene
Propene, also known as propylene or methylethylene, is an unsaturated organic compound having the chemical formula C3H6. It has one double bond, and is the second simplest member of the alkene class of hydrocarbons, and it is also second in natural abundance.-Properties:At room temperature and...
.
Olefin peroxidation
Most epoxides are generated by treating alkenes with peroxidePeroxide
A peroxide is a compound containing an oxygen–oxygen single bond or the peroxide anion .The O−O group is called the peroxide group or peroxo group. In contrast to oxide ions, the oxygen atoms in the peroxide ion have an oxidation state of −1.The simplest stable peroxide is hydrogen peroxide...
-containing reagents, which donate a single oxygen atom. Typical peroxide reagents include hydrogen peroxide, peroxycarboxylic acids (generated in-situ or preformed), and alkyl hydroperoxides. In specialized applications, other peroxide-containing reagents are employed, such as dimethyldioxirane
Dimethyldioxirane
Dimethyldioxirane is a dioxirane derived from acetone. It is the most commonly used dioxirane in organic synthesis.-Synthesis:DMDO is not commercially available because of its instability...
.
The largest scale application of this approach is the production of propylene oxide from propylene using either t-butyl hydroperoxide or ethylbenzene
Ethylbenzene
Ethylbenzene is an organic compound with the formula C6H5CH2CH3. This aromatic hydrocarbon is important in the petrochemical industry as an intermediate in the production of styrene, which in turn is used for making polystyrene, a common plastic material....
hydroperoxide. More typically for laboratory operations, the Prilezhaev reaction is employed. This approach involves the oxidation of the alkene with a peroxyacid such as m-CPBA
Meta-Chloroperoxybenzoic acid
meta-Chloroperoxybenzoic acid is a peroxycarboxylic acid used widely as an oxidant in organic synthesis. mCPBA is often preferred to other peroxy acids because of its relative ease of handling...
. Illustrative is the epoxidation of styrene with perbenzoic acid to styrene oxide
Styrene oxide
Styrene oxide is an epoxide derived from styrene. It may be prepared by epoxidation of styrene with peroxybenzoic acid, in the Prilezhaev reaction:- Toxicology :...
:
The reaction proceeds via what is commonly known as the "Butterfly Mechanism." The peroxide is viewed as an electrophile
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
, and the alkene a nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
. The reaction is considered to be concerted (the numbers in the mechanism below are for simplification).
Hydroperoxides are also employed in catalytic enantioselective epoxidations, such as the Sharpless epoxidation
Sharpless epoxidation
The Sharpless Epoxidation reaction is an enantioselective chemical reaction to prepare 2,3-epoxyalcohols from primary and secondary allylic alcohols....
and the Jacobsen epoxidation
Jacobsen epoxidation
The Jacobsen Epoxidation, sometimes also referred to as Jacobsen-Katsuki Epoxidation is a chemical reaction which allows enantioselective epoxidation of unfunctionalized alkyl- and aryl- substituted olefins. It is complementary to the Sharpless epoxidation...
. In such cases, the oxygen is delivered from a metal oxide or peroxide. Together with the Shi epoxidation
Shi epoxidation
The Shi epoxidation is a chemical reaction described as an asymmetric epoxidation of olefins with oxone and a fructose-derived catalyst ....
, these reactions are useful for the enantioselective synthesis of chiral epoxides. Oxaziridine
Oxaziridine
An oxaziridine is an organic molecule that features a three-membered heterocycle containing oxygen, nitrogen, and carbon.-History:Oxaziridine derivatives were first synthesized in the mid 1950s by Emmons and subsequently by Krimm and Horner and Jürgens...
reagents may also be used to generate epoxides from alkenes.
Intramolecular SN2 substitution
This method is a variant of the Williamson ether synthesisWilliamson ether synthesis
The Williamson ether synthesis is an organic reaction, forming an ether from an organohalide and an alcohol. This reaction was developed by Alexander Williamson in 1850. Typically it involves the reaction of an alkoxide ion with a primary alkyl halide via an SN2 reaction...
. In this case, an alkoxide ion displaces a chloride atom within the same molecule. The precursor compounds are called halohydrin
Halohydrin
A halohydrin or a haloalcohol is a type of organic compound or functional group in which one carbon atom has a halogen substituent, and an adjacent carbon atom has a hydroxyl substituent. They are derived from alcohols are therefore characterized by the presence of both the hydroxyl functional...
s. For example, with 2-chloropropanol:

Approximately, half of the world's supply of propylene oxide
Propylene oxide
Propylene oxide is an organic compound with the molecular formula CH3CHCH2O. This colourless volatile liquid is produced on a large scale industrially, its major application being its use for the production of polyether polyols for use in making polyurethane plastics...
arises via this route. An intramolecular epoxide formation reaction is one of the key steps in the Darzens reaction
Darzens reaction
The Darzens reaction is the chemical reaction of a ketone or aldehyde with an α-haloester to form an α,β-epoxy ester, also called a "glycidic ester"...
.
In the Johnson-Corey-Chaykovsky reaction
Johnson-Corey-Chaykovsky reaction
The Johnson–Corey–Chaykovsky reaction is a chemical reaction used in organic chemistry for the synthesis of epoxides, aziridines, and cyclopropanes. It was discovered in 1961 by A. William Johnson and developed significantly by E.J. Corey and Michael Chaykovsky...
epoxides are generated from carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
groups and sulfonium ylides. In this reaction, a sulfonium is the leaving group instead of chloride.
Nucleophilic epoxidation
Electron-deficient olefins, such as enoneEnone
An enone is an unsaturated chemical compound or functional group consisting of a conjugated system of an alkene and a ketone. The simplest enone is methyl vinyl ketone or CH2=CHCOCH3....
s and acryl derivatives
Acrylic acid
Acrylic acid is an organic compound with the formula CH2=CHCO2H. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a characteristic acrid or tart smell. It is miscible with water, alcohols,...
can be epoxidized using nucleophilic oxygen compounds such as peroxides. The reaction is a two-step mechanism. First the oxygen performs a nucleophilic conjugate addition
Nucleophilic conjugate addition
Nucleophilic conjugate addition is a type of organic reaction. Ordinary nucleophilic additions or 1,2-nucleophilic additions deal mostly with additions to carbonyl compounds. Simple alkene compounds do not show 1,2 reactivity due to lack of polarity, unless the alkene is activated with special...
to give a stabilized carbanion. This carbanion then attacks the same oxygen atom, displacing a leaving group from it, to close the epoxide ring.
Asymmetric epoxidation
The carbon atoms of an epoxide are approximately sp3-hybridizedOrbital hybridisation
In chemistry, hybridisation is the concept of mixing atomic orbitals to form new hybrid orbitals suitable for the qualitative description of atomic bonding properties. Hybridised orbitals are very useful in the explanation of the shape of molecular orbitals for molecules. It is an integral part...
, and thus may be stereogenic positions. Depending on the mechanism of the reaction and the geometry of the alkene starting material, cis and/or trans epoxide diastereomer
Diastereomer
Diastereomers are stereoisomers that are not enantiomers.Diastereomerism occurs when two or more stereoisomers of a compound have different configurations at one or more of the equivalent stereocenters and are not mirror images of each other.When two diastereoisomers differ from each other at...
s may be formed. In addition, if there are other stereocenters present in the starting material, they can influence the stereochemistry of the epoxidation relative to them. This diastereoselectivity is a form of "substrate control" of the reaction. Finally, epoxidizing agents that possess stereogenic structures can influence the stereochemistry of the epoxide product (see for example the Sharpless epoxidation
Sharpless epoxidation
The Sharpless Epoxidation reaction is an enantioselective chemical reaction to prepare 2,3-epoxyalcohols from primary and secondary allylic alcohols....
, Jacobsen epoxidation
Jacobsen epoxidation
The Jacobsen Epoxidation, sometimes also referred to as Jacobsen-Katsuki Epoxidation is a chemical reaction which allows enantioselective epoxidation of unfunctionalized alkyl- and aryl- substituted olefins. It is complementary to the Sharpless epoxidation...
, and Juliá-Colonna Epoxidation
Juliá-Colonna epoxidation
The Juliá-Colonna epoxidation is an asymmetric poly-leucine catalyzed nucleophilic epoxidation of electron deficient olefins in a triphasic system...
). This enantioselectivity is a form of "reagent control" of the reaction.
Reactions
Typical epoxide reactions are listed below.- Nucleophilic additionNucleophilic additionIn organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile....
to an epoxide can be base or acid catalyzed.
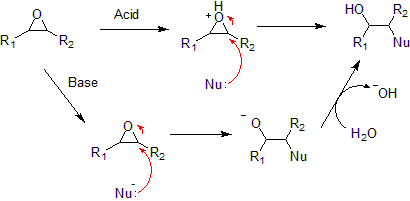
- Under acidic conditions, the nucleophile attacks the carbon that will form the most stable carbocationCarbocationA carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
, i.e. the most substituted carbon (similar to a halonium ion). Under basic conditions, the nucleophile attacks the least substituted carbon, in accordance with standard SN2 nucleophilic addition reaction process.
- Under acidic conditions, the nucleophile attacks the carbon that will form the most stable carbocation
- HydrolysisHydrolysisHydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
of an epoxide in presence of an acid catalyst generates a glycol. The hydrolysisHydrolysisHydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
process of epoxides can be considered to be the nucleophilic additionNucleophilic additionIn organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile....
of water to the epoxide under acidicAcid catalysisIn acid catalysis and base catalysis a chemical reaction is catalyzed by an acid or a base. The acid is often the proton and the base is often a hydroxyl ion. Typical reactions catalyzed by proton transfer are esterfications and aldol reactions. In these reactions the conjugate acid of the carbonyl...
conditions. - Reduction of an epoxide with lithium aluminium hydrideLithium aluminium hydrideLithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
and waterWaterWater is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
generates an alcoholAlcoholIn chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
. This reduction process can be considered to be the nucleophilic addition of hydride (H-) to the epoxide under basic conditions.
- Reduction with tungsten hexachlorideTungsten hexachlorideTungsten hexachloride is the chemical compound of tungsten and chlorine with the formula WCl6. This dark violet blue species exists as a volatile solid under standard conditions. It is an important starting reagent in the preparation of tungsten compounds. WCl6 is a rare example of a...
and n-butyllithiumN-Butyllithiumn-Butyllithium is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene...
generates the alkeneAlkeneIn organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
. This reaction in effect is a de-epoxidation:
- Reaction with the NH group in an amineAmineAmines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
. This covalent bond formation is utilised in epoxyEpoxyEpoxy, also known as polyepoxide, is a thermosetting polymer formed from reaction of an epoxide "resin" with polyamine "hardener". Epoxy has a wide range of applications, including fiber-reinforced plastic materials and general purpose adhesives....
glue with, e.g., Triethylenetetramine (TETA) as a hardener.
Perepoxides
Perepoxides are epoxides with an additional oxygen atom attached to the epoxide-oxygen. They are isoelectronic and isostructural with the cyclic sulfoxides derived from episulfideEpisulfide
Episulfides are a class of organic compounds that contain a saturated heterocyclic ring consisting of two carbon atoms and one sulfur atom. It is the sulfur analogue of an epoxide or aziridine. They are also known as thiiranes, olefin sulfides, thioalkylene oxides, and thiacyclopropanes.The parent...
s. Perepoxides are proposed intermediates in the photosensitized oxidation of alkenes, as occurs when drying oil
Drying oil
A drying oil is an oil that hardens to a tough, solid film after a period of exposure to air. The oil hardens through a chemical reaction in which the components crosslink by the action of oxygen . Drying oils are a key component of oil paint and some varnishes...
s (a component of some paints and varnishes) are exposed to air in light. Such intermediates arise from the addition of singlet oxygen
Singlet oxygen
Singlet oxygen is the common name used for the diamagnetic form of molecular oxygen , which is less stable than the normal triplet oxygen. Because of its unusual properties, singlet oxygen can persist for over an hour at room temperature, depending on the environment...
to the double bond. Perepoxides rapidly rearrange to allylic hydroperoxides.
See also
- Polyether
- Epoxide hydrolaseEpoxide hydrolaseEpoxide hydrolase functions in detoxication during drug metabolism. It converts epoxides to trans-dihydrodiols, which can be conjugated and excreted from the body. Epoxides result from the degradation of aromatic compounds...
- Juliá-Colonna epoxidationJuliá-Colonna epoxidationThe Juliá-Colonna epoxidation is an asymmetric poly-leucine catalyzed nucleophilic epoxidation of electron deficient olefins in a triphasic system...
- Johnson-Corey-Chaykovsky reactionJohnson-Corey-Chaykovsky reactionThe Johnson–Corey–Chaykovsky reaction is a chemical reaction used in organic chemistry for the synthesis of epoxides, aziridines, and cyclopropanes. It was discovered in 1961 by A. William Johnson and developed significantly by E.J. Corey and Michael Chaykovsky...