Electrophilic aromatic substitution
Overview
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
in which an atom, usually hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
, appended to an aromatic system is replaced by an electrophile
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
. The most important reactions of this type that take place are aromatic nitration, aromatic halogenation, aromatic sulfonation
Aromatic sulfonation
Aromatic sulfonation is an organic reaction in which a hydrogen atom on an arene is replaced by a sulfonic acid functional group in an electrophilic aromatic substitution.-Mechanism:...
, and acylation and alkylating Friedel–Crafts reactions.
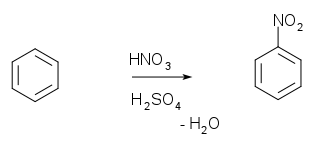
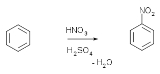
Aromatic nitrations to form nitro compound
Nitro compound
Nitro compounds are organic compounds that contain one or more nitro functional groups . They are often highly explosive, especially when the compound contains more than one nitro group and is impure. The nitro group is one of the most common explosophores used globally...
s take place by generating a nitronium ion from nitric acid
Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
and sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
.
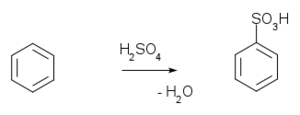
Aromatic sulfonation
Aromatic sulfonation
Aromatic sulfonation is an organic reaction in which a hydrogen atom on an arene is replaced by a sulfonic acid functional group in an electrophilic aromatic substitution.-Mechanism:...
of benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
with fuming sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
gives benzenesulfonic acid.
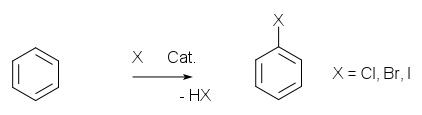
Aromatic halogenation of benzene with bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
, chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
or iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
gives the corresponding aryl halogen compounds catalyzed by the corresponding iron trihalide.
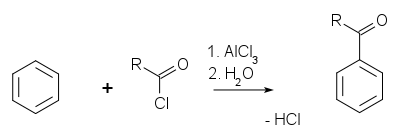
The Friedel-Crafts reaction
Friedel-Crafts reaction
The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877. There are two main types of Friedel–Crafts reactions: alkylation reactions and acylation reactions. This reaction type is a form of electrophilic aromatic substitution...
exists as an acylation
Acylation
In chemistry, acylation is the process of adding an acyl group to a compound. The compound providing the acyl group is called the acylating agent....
and an alkylation
Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion or a carbene . Alkylating agents are widely used in chemistry because the alkyl group is probably the most common group encountered in...
with acyl halides or alkyl halides as reactants.
The catalyst is most typically aluminium trichloride, but almost any strong Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
can be used.
Unanswered Questions