Alkyne
Encyclopedia
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
s that have a triple bond
Triple bond
A triple bond in chemistry is a chemical bond between two chemical elements involving six bonding electrons instead of the usual two in a covalent single bond. The most common triple bond, that between two carbon atoms, can be found in alkynes. Other functional groups containing a triple bond are...
between two carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic but tend to be more reactive.
Chemical properties
Alkynes are characteristically more unsaturatedSaturation (chemistry)
In chemistry, saturation has six different meanings, all based on reaching a maximum capacity...
than alkenes. Thus they add two equivalents of bromine whereas an alkene adds only one equivalent. Other reactions are listed below. Alkynes are usually more reactive than alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s. They show greater tendency to polymerize
Polymerization
In polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains...
or oligomerize
Oligomer
In chemistry, an oligomer is a molecule that consists of a few monomer units , in contrast to a polymer that, at least in principle, consists of an unlimited number of monomers. Dimers, trimers, and tetramers are oligomers. Many oils are oligomeric, such as liquid paraffin...
than alkenes do. The resulting polymers, called polyacetylene
Polyacetylene
Polyacetylene is an organic polymer with the repeat unit n. The high electrical conductivity discovered for these polymers beginning in the 1960's accelerated interest in the use of organic compounds in microelectronics...
s (which do not contain alkyne units) are conjugated
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
and can exhibit semiconducting properties.
Structure and bonding
In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes tend to be rod-like. Correspondingly, cyclic alkynes are rare. Benzyne is highly unstable. The C≡C bond distance of 121 picometers is much shorter than the C=C distance in alkeneAlkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s (134 pm) or the C-C bond in alkanes (153 pm).
The triple bond
Triple bond
A triple bond in chemistry is a chemical bond between two chemical elements involving six bonding electrons instead of the usual two in a covalent single bond. The most common triple bond, that between two carbon atoms, can be found in alkynes. Other functional groups containing a triple bond are...
is very strong with a bond strength
Bond strength
In chemistry, bond strength is measured between two atoms joined in a chemical bond. It is the degree to which each atom linked to another atom contributes to the valency of this other atom...
of 839 kJ/mol. The sigma bond contributes 369 kJ/mol, the first pi bond contributes 268 kJ/mol and the second pi-bond of 202 kJ/mol bond strength. Bonding usually discussed in the context of molecular orbital theory
Molecular orbital theory
In chemistry, molecular orbital theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the whole molecule...
, which recognizes the triple bond as arising from overlap of s and p orbitals. In the language of valence bond theory
Valence bond theory
In chemistry, valence bond theory is one of two basic theories, along with molecular orbital theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of the dissociated atoms combine to give individual chemical bonds...
, the carbon atoms in an alkyne bond are sp hybridized: they each have two unhybridized p orbitals and two sp hybrid orbitals
Orbital hybridisation
In chemistry, hybridisation is the concept of mixing atomic orbitals to form new hybrid orbitals suitable for the qualitative description of atomic bonding properties. Hybridised orbitals are very useful in the explanation of the shape of molecular orbitals for molecules. It is an integral part...
. Overlap of an sp orbital from each atom forms one sp-sp sigma bond
Sigma bond
In chemistry, sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most clearly defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a σ-bond is...
. Each p orbital on one atom overlaps one on the other atom, forming two pi bond
Pi bond
In chemistry, pi bonds are covalent chemical bonds where two lobes of one involved atomic orbital overlap two lobes of the other involved atomic orbital...
s, giving a total of three bonds. The remaining sp orbital on each atom can form a sigma bond to another atom, for example to hydrogen atoms in the parent acetylene]. The two sp orbitals project on opposite sides of the carbon atom.
Terminal and internal alkynes
Internal alkynes feature carbon substituents on each acetylenic carbon. Symmetrical examples include diphenylacetyleneDiphenylacetylene
Diphenylacetylene is the chemical compound C6H5C≡CC6H5. The molecule consists of phenyl groups attached to both ends of an alkyne. It is a colorless crystalline material that is widely used as a building block in organic and as a ligand in organometallic chemistry.-Preparation:Several...
and 3-hexyne
3-Hexyne
3-Hexyne is the organic compound with the formula C2H5CCC2H5. This colorless liquid is the most common of the three isomeric hexynes. Together with 2-butyne and diphenylacetylene, it serves as a reference acetylenic ligand in organometallic chemistry...
. A representative unsymmetrical internal alkyne is propiolic acid
Propiolic acid
Propiolic acid, or acetylene mono-carboxylic acid, is an unsaturated organic acid prepared by boiling acetylenedicarboxylic acid, obtained by the action of alcoholic potash on dibromosuccinic acid, or its acid potassium salt with water. It forms silky crystals which melt at 9 °C, and boil at about...
. Terminal alkynes have at least one hydrogen atom bonded to an sp hybridized carbon (those involved in the triple bond. An example would be methylacetylene
Methylacetylene
Methylacetylene is an alkyne with the chemical formula H3C≡CH. It is a component of MAPP gas along with its isomer 1,2-propadiene , which is commonly used in gas welding...
(propyne using IUPAC nomenclature). Terminal alkynes and acetylene
Acetylene
Acetylene is the chemical compound with the formula C2H2. It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in pure form and thus is usually handled as a solution.As an alkyne, acetylene is unsaturated because...
are mildly acidic. The acidic hydrogen on terminal alkynes can be replaced by a variety of groups resulting in halo-, silyl-, and alkoxoalkynes.
Synthesis
Commercially, the dominant alkyne is acetylene itself, which is used as a fuel and a precursor to other compounds, e.g., acrylates. Hundreds of millions of kilograms are produced annually by dehydrogenation of natural gasNatural gas
Natural gas is a naturally occurring gas mixture consisting primarily of methane, typically with 0–20% higher hydrocarbons . It is found associated with other hydrocarbon fuel, in coal beds, as methane clathrates, and is an important fuel source and a major feedstock for fertilizers.Most natural...
:
- 2 CH4 → HC≡CH + 3 H2
Propyne, also industrially useful, is also prepared by thermal cracking of hydrocarbons. Most other industrially useful alkyne derivatives are prepared from acetylene, e.g. via condensation with formaldehyde.
Specialty alkynes are prepared by dehydrohalogenation
Dehydrohalogenation
Dehydrohalogenation is an organic reaction from which an alkene is obtained from an alkyl halide . It is also called a β-Elimination reaction and is a type of elimination reaction....
of vicinal
Vicinal (chemistry)
In chemistry vicinal stands for any two functional groups bonded to two adjacent carbon atoms. For example the molecule 2,3-dibromobutane carries two vicinal bromine atoms and 1,3-dibromobutane does not....
alkyl dihalides
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
or vinyl halides. Metal acetylides can be coupled with primary alkyl halides. Via the Fritsch-Buttenberg-Wiechell rearrangement
Fritsch-Buttenberg-Wiechell rearrangement
The Fritsch–Buttenberg–Wiechell rearrangement, named for Paul Ernst Moritz Fritsch, Wilhelm Paul Buttenberg, and Heinrich G. Wiechell, is a chemical reaction whereby a 1,1-diaryl-2-bromo-alkene rearranges to a 1,2-diaryl-alkyne by reaction with a strong base such as an alkoxide.This rearrangement...
, alkynes are prepared from vinyl bromide
Vinyl bromide
Vinyl bromide is a simple vinyl halide. It is soluble in chloroform, ethanol, diethyl ether, acetone and benzene.- Uses :Vinyl bromide is used to manufacture bromopolymers and mainly polyvinyl bromide...
s. Alkynes can be prepared from aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s using the Corey-Fuchs reaction
Corey-Fuchs reaction
The Corey–Fuchs reaction, also known as the Ramirez–Corey–Fuchs reaction, is a series of chemical reactions designed to transform an aldehyde into an alkyne. The formation of the 1,1-dibromoolefins via phosphine-dibromomethylenes was originally discovered by Desai, McKelvie and Ramirez...
and from aldehydes or ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s by the Seyferth–Gilbert homologation. In the alkyne zipper reaction
Alkyne zipper reaction
The alkyne zipper reaction is an organic reaction which isomerizes an organic compound containing an internal alkyne into a terminal alkyne. This was first reported by Charles Allen Brown and Ayako Yamashita in 1975...
, alkynes are generated from other alkynes by treatment with a strong base.
Reactions
Featuring a reactive functional groupFunctional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
, alkynes participate in many organic reaction
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
s.
Addition of hydrogen, halogens, and related reagents
Alkynes characteristically undergo reactions that show that they are "doubly unsaturated," meaning that each alkyne unit is capable of adding two equivalents of H2, halogenHalogen
The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
s or related HX reagents (X = halide, pseudohalide, etc.). Depending on catalysts and conditions, alkynes add one or two equivalents of hydrogen. Hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
to the alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
is usually more desirable since alkanes are less useful:
- RC≡CR' + H2 → cis-RCH=CR'H
The largest scale application of this technology is the conversion of acetylene to ethylene in refineries. The steam cracking of alkanes affords a few percent acetylene, which is selectively hydrogenated in the presence of a palladium
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
/silver
Silver
Silver is a metallic chemical element with the chemical symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal...
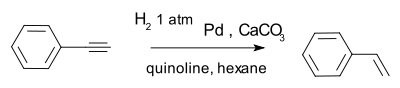
catalyst. For more complex alkynes, the Lindlar catalyst
Lindlar catalyst
A Lindlar catalyst is a heterogeneous catalyst that consists of palladium deposited on calcium carbonate and treated with various forms of lead. The lead additive serves to deactivate the palladium sites. A variety of "catalyst poisons" have been used including lead acetate and lead oxide. The...
is widely recommended to avoid formation of the alkane, for example in the conversion of phenylacetylene
Phenylacetylene
Phenylacetylene is an alkyne hydrocarbon containing a phenyl group. It exists as a colorless, viscous liquid. In research, it is sometimes used as an analog for acetylene; being a liquid, it is easier to handle than acetylene gas.-Preparation:...
to styrene.
Similarly, halogenation
Halogenation
Halogenation is a chemical reaction that incorporates a halogen atom into a molecule in substitution of hydrogen atom. Halogenation takes place in the gas phase. There are four types of halogenation: fluorination, chlorination, bromination, and iodination...
of alkynes gives the vinyl dihalides or alkyl tetrahalides:
- RC≡CR' + 2 Br2 → RCBr2CRBr2
The addition of nonpolar E-H bonds across C≡C is general for silanes, boranes, and related hydrides. The hydroboration
Hydroboration-oxidation reaction
In organic chemistry, the hydroboration–oxidation reaction is a two-step organic reaction that converts an alkene into a neutral alcohol by the net addition of water across the double bond. The hydrogen and hydroxyl group are added in a syn addition leading to cis stereochemistry...
of alkynes gives vinylic boranes which oxidize to the corresponding aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
or ketone. In the thiol-yne reaction
Thiol-yne reaction
The Thiol-yne reaction is an organic reaction between a thiol and an alkyne. The reaction product is an alkenyl sulfide...
the substrate is a thiol.
Hydrohalogenation
Hydrohalogenation
A hydrohalogenation reaction is the electrophilic addition of hydrohalic acids like hydrogen chloride or hydrogen bromide to alkenes to yield the corresponding haloalkanes....
gives the corresponding vinyl halide
Vinyl halide
In organic chemistry, a vinyl halide is any alkene with at least one halide substituent bonded directly on one of the unsaturated carbons. Vinyl chloride is one such substance....
s or alkyl dihalides, again depending on the number of equivalents of HX added. The addition of water to alkynes is a related reaction except the initial enol
Enol
Enols are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated anions of enols are called enolates...
intermediate converts to the ketone or aldehyde. Illustrative is the hydration of phenylacetylene
Phenylacetylene
Phenylacetylene is an alkyne hydrocarbon containing a phenyl group. It exists as a colorless, viscous liquid. In research, it is sometimes used as an analog for acetylene; being a liquid, it is easier to handle than acetylene gas.-Preparation:...
gives acetophenone
Acetophenone
Acetophenone is the organic compound with the formula C6H5CCH3. It is the simplest aromatic ketone. This colourless, viscous liquid is a precursor to useful resins and fragrances.-Production:Acetophenone can be obtained by a variety of methods...
, and the (Ph3P)AuCH3-catalyzed hydration of 1,8-nonadiyne to 2,8-nonanedione:
- PhC≡CH + H2O → PhCOCH3
- HC≡CC6H12C≡CH + 2H2O → CH3COC6H12COCH3
Cycloadditions and oxidation
Alkynes undergo diverse cycloadditionCycloaddition
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
reactions. Most notable is the Diels–Alder reaction with 1,3-diene
Diene
In organic chemistry a diene or diolefin is a hydrocarbon that contains two carbon double bonds.Conjugated dienes are functional groups, with a general formula of CnH2n-2. Dienes and alkynes are functional isomers...
s to give 1,4-cyclohexadiene
Cyclohexadiene
-See also:* Benzene or its theoretical isomer 1,3,5-Cyclohexatriene* Cyclohexene...
s. This general reaction has been extensively developed and electrophilic alkynes are especially effective dienophiles. The "cycloadduct" derived from the addition of alkynes to 2-pyrone
2-Pyrone
2-Pyrone is an unsaturated cyclic chemical compound with the molecular formula C5H4O2. It is isomeric with 4-pyrone....
eliminates carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
to give the aromatic compound. Other specialized cycloadditions include multicomponent reactions such as alkyne trimerisation
Alkyne trimerisation
An alkyne trimerisation reaction is a 2+2+2 cyclization reaction in which three alkyne molecules react to form an aromatic compound. The reaction is 'pseudo' pericyclic since it has not been observed to occur without the assistance of metal catalysis; and the metal catalyst assembles the ring...
to give aromatic compounds and the [2+2+1]cycloaddition of an alkyne, alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
and carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
in the Pauson–Khand reaction
Pauson–Khand reaction
The Pauson–Khand reaction is a chemical reaction described as a [2+2+1] cycloaddition between an alkyne, an alkene and carbon monoxide to form a α,β-cyclopentenone...
. Non-carbon reagents also undergo cyclization, e.g. Azide alkyne Huisgen cycloaddition
Azide alkyne Huisgen cycloaddition
The Azide-Alkyne Huisgen Cycloaddition is a 1,3-dipolar cycloaddition between an azide and a terminal or internal alkyne to give a 1,2,3-triazole. Rolf Huisgen was the first to understand the scope of this organic reaction. American chemist K...
to give triazole
Triazole
Triazole refers to either one of a pair of isomeric chemical compounds with molecular formula C2H3N3, having a five-membered ring of two carbon atoms and three nitrogen atoms.The two isomers are:*1,2,3-Triazole 100px*1,2,4-Triazole 100px...
s. Cycloaddition processes involving alkynes are often catalyzed by metals, e.g. enyne metathesis
Enyne metathesis
An Enyne metathesis is an organic reaction taking place between an alkyne and an alkene with a metal carbene catalyst forming a butadiene. This reaction is a variation of olefin metathesis.The general scheme is given by scheme 1:...
and alkyne metathesis
Alkyne metathesis
Alkyne metathesis is an organic reaction involving the redistribution of alkyne chemical bonds. This reaction is closely related to olefin metathesis. Metal-catalyzed alkyne metathesis was first described in 1968 by Bailey, et al. The Bailey system utilized a mixture of tungsten and silicon oxides...
, which allows the scrambling of carbyne (RC) centers:
- RC≡CR + R'C≡CR'
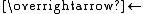 2 RC≡CR'
2 RC≡CR'
Oxidative cleavage of alkynes proceeds via cycloaddition to metal oxides. Most famously, potassium permanganate
Potassium permanganate
Potassium permanganate is an inorganic chemical compound with the formula KMnO4. It is a salt consisting of K+ and MnO4− ions. Formerly known as permanganate of potash or Condy's crystals, it is a strong oxidizing agent. It dissolves in water to give intensely purple solutions, the...
converts alkynes to a pair of carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
s.
Reactions specific for terminal alkynes
In addition to undergoing the reactions characteristic of internal alkynes, terminal alkynes are reactive as weak acids, with pKaAcid dissociation constant
An acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
values (25) between that of ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
(35) and ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
(16). The acetylide conjugate base is stabilized as a result of the high s character of the sp orbital, in which the electron pair resides. Electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s in an s orbital benefit from closer proximity to the positively charged atom nucleus, and are therefore lower in energy. Treatment of terminal alkynes with a strong base gives the corresponding metal acetylide
Metal acetylide
Acetylide, ethynide, dicarbide, or percarbide is the divalent anion with formula C22− or 2−. It may be regarded as the result of removing two protons from acetylene C2H2 or H-C≡C-H, the prototypical alkyne — that behaves as a weak acid.These terms are also used for any monovalent anion...
s:
- RC≡CH + MX → RC≡CM + HX (MX = NaNH2Sodium amideSodium amide, commonly called sodamide, is the chemical compound with the formula NaNH2. This solid, which is dangerously reactive toward water, is white when pure, but commercial samples are typically gray due to the presence of small quantities of metallic iron from the manufacturing process...
, LiBuN-Butyllithiumn-Butyllithium is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene...
, RMgX)
The reactions of alkynes with certain metal cations, e.g. Ag+ also gives acetylides. Thus, few drops of diamminesilver(I) hydroxide (Ag(NH3)2OH) reacts with terminal alkynes signaled by formation of a white precipitate of the silver acetylide.
Acetylide derivatives are synthetically useful nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
s that participate in C-C bond forming reactions, as illustrated in the area called "Reppe Chemistry"
Carbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry.-Organic chemistry:...
.
In the Favorskii reaction
Favorskii reaction
The Favorskii reaction , named for the Russian chemist Alexei Yevgrafovich Favorskii, is a special case of nucleophilic attack on a carbonyl group involving a terminal alkyne with acidic protons....
, terminal alkynes add to carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
compounds to give the hydroxyalkyne. Coupling
Coupling reaction
A coupling reaction in organic chemistry is a catch-all term for a variety of reactions where two hydrocarbon fragments are coupled with the aid of a metal catalyst...
of terminal alkynes to give di-alkynes is effected in the Cadiot-Chodkiewicz coupling
Cadiot-Chodkiewicz coupling
The Cadiot-Chodkiewicz coupling in organic chemistry is a coupling reaction between a terminal alkyne and a haloalkyne catalyzed by a copper salt such as copper bromide and an amine base. The reaction product is a di-acetylene or di-alkyne....
, Glaser coupling, and the Eglinton coupling reactions. Terminal alkynes can also be coupled to aryl or vinyl halides as in the Sonogashira coupling
Sonogashira coupling
In organic chemistry, a Sonogashira coupling is a coupling reaction of terminal alkynes with aryl or vinyl halides. This reaction was first reported by Kenkichi Sonogashira and Nobue Hagihara in 1975.-Catalyst:...
.
Alkynes in nature and medicine
According to Ferdinand Bohlmann, the first naturally occurring acetylenic compound, dehydromatricaria ester, was isolated from an Artemisia species in 1826. In the nearly two centuries that have followed, well over a thousand naturally occurring acetylenes have been discovered and reported. Polyynes,a subset of this class of natural products, have been isolated from a wide variety of plant species,cultures of higher fungi, bacteria, marine sponges, and corals. Some acids like tariric acid contains an alkyne group. Diynes and triynes, species with the linkage RC≡C-C≡CR' and RC≡C-C≡C-C≡CR' respectively, occur in certain plants (IchthyothereIchthyothere
Ichthyothere is a small genus of about 25 flowering plants, found in parts of South America and Central America.The name ichthyothere literally translates as fish poison...
, Chrysanthemum
Chrysanthemum
Chrysanthemums, often called mums or chrysanths, are of the genus constituting approximately 30 species of perennial flowering plants in the family Asteraceae which is native to Asia and northeastern Europe.-Etymology:...
, Cicuta
Cicuta
Cicuta, commonly known as water hemlock, is a small genus of four species of highly poisonous plants in the family Apiaceae. They are perennial herbaceous plants which grow up to tall, having distinctive small green or white flowers arranged in an umbrella shape . Plants in this genus may also be...
, Oenanthe
Oenanthe
-The name of two genera:* Oenanthe, the Wheatear genus of birds* Oenanthe, the Water dropwort genus of plants-Persons:* Oenanthe of Egypt , an Egyptian Greek noblewoman and the wife of Agathocles...
and other members of the Asteraceae
Asteraceae
The Asteraceae or Compositae , is an exceedingly large and widespread family of vascular plants. The group has more than 22,750 currently accepted species, spread across 1620 genera and 12 subfamilies...
and Apiaceae
Apiaceae
The Apiaceae , commonly known as carrot or parsley family, is a group of mostly aromatic plants with hollow stems. The family is large, with more than 3,700 species spread across 434 genera, it is the sixteenth largest family of flowering plants...
families). Some examples are cicutoxin
Cicutoxin
Cicutoxin is a poisonous polyyne and alcohol found in various plants, most notably water hemlock . It is structurally related to the oenanthotoxin of hemlock water dropwort....
, oenanthotoxin
Oenanthotoxin
Oenanthotoxin is a toxin extracted from hemlock water dropwort and other plants of the genus Oenanthe. It is a central nervous system poison, and acts as a noncompetitive gamma-aminobutyric acid antagonist. This toxin played some role in euthanasia in ancient Sardinia, for inducing risus...
, falcarinol
Falcarinol
Falcarinol is a natural pesticide and fatty alcohol found in carrots, red ginseng and ivy. It protects roots from fungal diseases, such as liquorice rot that causes black spots on the roots during storage. Falcarinol is a polyyne with two carbon carbon triple bonds and two double bonds...
and carotatoxin
Carotatoxin
Carotatoxin is a poisonous polyyne and alcohol found in carrot . It is structurally related to the oenanthotoxin and cicutoxin. It occurs approximately in a concentration of 2 mg/kg....
. These compounds are highly bioactive, e.g. as nematocides. 1-Phenylhepta-1,3,5-triyne is illustrative of a naturally occurring triyne.
Alkynes occur in some pharmaceuticals, including the contraceptive norethynodrel
Norethynodrel
Norethynodrel was the progestin used in Enovid, the first oral contraceptive....
. A carbon-carbon triple bond is also present in marketed drugs such as the antiretroviral Efavirenz
Efavirenz
Efavirenz is a non-nucleoside reverse transcriptase inhibitor and is used as part of highly active antiretroviral therapy for the treatment of a human immunodeficiency virus type 1....
and the antifungal Terbinafine
Terbinafine
Terbinafine hydrochloride is a...
. Molecules called ene-diynes feature a ring containing an alkene ("ene") between two alkyne groups ("diyne"). These compounds, e.g. calicheamicin
Calicheamicin
The calicheamicins are a class of enediyne antibiotics derived from the bacterium Micromonospora echinospora, with calicheamicin γ1 being the most notable. It was isolated originally from a rock collected by a Scripps Research Institute chemist while hiking in Waco, Texas. It is extremely toxic to...
, are some of the most aggressive antitumor drugs known, so much so that the ene-diyne subunit is sometimes referred to as a "warhead." Ene-diynes undergo rearrangement via the Bergman cyclization
Bergman cyclization
The Bergman cyclization or Bergman reaction or Bergman cycloaromatization is an organic reaction and more specifically a rearrangement reaction taking place when an enediyne is heated in presence of a suitable hydrogen donor . It is named for the American chemist Robert George Bergman...
, generating highly reactive radical intermediates that attack DNA within the tumor.