
Reactivity–selectivity principle
Encyclopedia
In chemistry
the reactivity–selectivity principle or RSP states that a more reactive chemical compound
or reactive intermediate
is less selective in chemical reactions. In this context selectivity represents the ratio of reaction rate
s.
This principle was generally accepted until the 1970s when too many exceptions started to appear. The principle is now considered obsolete .
A classic example of perceived RSP found in older organic textbooks concerns the free radical halogenation
of simple alkane
s. Whereas the relatively unreactive bromine
reacts with 2-methylbutane predominantly to 2-bromo-2-methylbutane, the reaction with much more reactive chlorine
results in a mixture of all four regioisomers.
Another example of RSP can be found in the selectivity of the reaction of certain carbocation
s with azide
s and water
. The very stable triphenylmethyl carbocation derived from solvolysis
of the corresponding triphenylmethyl chloride
reacts 100 times faster with the azide anion than with water. When the carbocation is the very reactive tertiary adamantane
carbocation (as judged from diminished rate
of solvolysis) this difference is only a factor of 10.
Constant or inverse relationships are just as frequent. For example a group of 3- and 4-substituted pyridine
s in their reactivity quantified by their pKa
show the same selectivity in their reactions with a group of alkylating reagents.
The reason for the early success of RSP was that the experiments involved very reactive intermediates with reactivities close to kinetic diffusion control and as a result the more reactive intermediate appeared to react slower with the faster substrate.
General relationships between reactivity and selectivity in chemical reactions can successfully explained by Hammond's postulate
.
When reactivity-selectivity relationships do exist they signify different reaction modes. In one study the reactivity of two different free radical species (A, sulfur, B carbon) towards addition to simple alkene
s such as acrylonitrile
, vinyl acetate
and acrylamide
was examined.
The sulfur radical was found to be more reactive (6*108 vs. 1*107 mole-1.s-1) and less selective (selectivity ratio 76 vs 1200) than the carbon radical. In this case the effect can be explained by extending the Bell–Evans–Polanyi principle
with a factor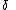 accounting for transfer of charge from the reactants to the transition state
accounting for transfer of charge from the reactants to the transition state
of the reaction which can be calculated in silico
:
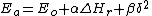
with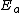 the activation energy
the activation energy
and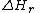 the reaction enthalpy
the reaction enthalpy
change. With the electrophilic
sulfur radical the charge transfer is largest with electron-rich alkenes such as acrylonitrile but the resulting reduction in activation energy (β is negative) is offset by a reduced enthalpy. With the nucleophilic
carbon radical on the other hand both enthalpy and polar effects have the same direction thus extending the activation energy range.
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
the reactivity–selectivity principle or RSP states that a more reactive chemical compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
or reactive intermediate
Reactive intermediate
In chemistry a reactive intermediate is a short-lived, high energy, highly reactive molecule. When generated in a chemical reaction it will quickly convert into a more stable molecule. Only in exceptional cases can these compounds be isolated and stored, e.g. low temperatures, matrix isolation...
is less selective in chemical reactions. In this context selectivity represents the ratio of reaction rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
s.
This principle was generally accepted until the 1970s when too many exceptions started to appear. The principle is now considered obsolete .
A classic example of perceived RSP found in older organic textbooks concerns the free radical halogenation
Free radical halogenation
In organic chemistry, free-radical halogenation is a type of halogenation. This chemical reaction is typical of alkanes and alkyl-substituted aromatics under application of heat or UV light. The reaction is used for the industrial synthesis of chloroform , dichloromethane , and hexachlorobutadiene...
of simple alkane
Alkane
Alkanes are chemical compounds that consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds without any cycles...
s. Whereas the relatively unreactive bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
reacts with 2-methylbutane predominantly to 2-bromo-2-methylbutane, the reaction with much more reactive chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
results in a mixture of all four regioisomers.
Another example of RSP can be found in the selectivity of the reaction of certain carbocation
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
s with azide
Azide
Azide is the anion with the formula N3−. It is the conjugate base of hydrazoic acid. N3− is a linear anion that is isoelectronic with CO2 and N2O. Per valence bond theory, azide can be described by several resonance structures, an important one being N−=N+=N−...
s and water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
. The very stable triphenylmethyl carbocation derived from solvolysis
Solvolysis
Solvolysis is a special type of nucleophilic substitution or elimination where the nucleophile is a solvent molecule. For certain nucleophiles, there are specific terms for the type of solvolysis reaction...
of the corresponding triphenylmethyl chloride
Triphenylmethyl chloride
Triphenylmethyl chloride or trityl chloride is a white solid with the chemical formula C19H15Cl. It is an alkyl halide, sometimes used to introduce the trityl protecting group.-Preparation:Triphenylmethyl chloride is commercially available...
reacts 100 times faster with the azide anion than with water. When the carbocation is the very reactive tertiary adamantane
Adamantane
Adamantane is a colorless, crystalline chemical compound with a camphor-like odor. With a formula C10H16, it is a cycloalkane and also the simplest diamondoid. Adamantane molecules consist of three cyclohexane rings arranged in the "armchair" configuration. It is unique in that it is both rigid...
carbocation (as judged from diminished rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
of solvolysis) this difference is only a factor of 10.
Constant or inverse relationships are just as frequent. For example a group of 3- and 4-substituted pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
s in their reactivity quantified by their pKa
PKA
PKA, pKa, or other similar variations may stand for:* pKa, the symbol for the acid dissociation constant at logarithmic scale* Protein kinase A, a class of cAMP-dependent enzymes* Pi Kappa Alpha, the North-American social fraternity...
show the same selectivity in their reactions with a group of alkylating reagents.
The reason for the early success of RSP was that the experiments involved very reactive intermediates with reactivities close to kinetic diffusion control and as a result the more reactive intermediate appeared to react slower with the faster substrate.
General relationships between reactivity and selectivity in chemical reactions can successfully explained by Hammond's postulate
Hammond's Postulate
Hammond's postulate, also referred to as the Hammond–Leffler postulate, is a hypothesis, derived from transition state theory, concerning the transition state of organic chemical reactions, which states that:-Interpreting the postulate:...
.
When reactivity-selectivity relationships do exist they signify different reaction modes. In one study the reactivity of two different free radical species (A, sulfur, B carbon) towards addition to simple alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s such as acrylonitrile
Acrylonitrile
Acrylonitrile is the chemical compound with the formula C3H3N. This pungent-smelling colorless liquid often appears yellow due to impurities. It is an important monomer for the manufacture of useful plastics. In terms of its molecular structure, it consists of a vinyl group linked to a nitrile...
, vinyl acetate
Vinyl acetate
Vinyl acetate is an organic compound with the formula CH3COOCH=CH2. A colorless liquid with a pungent odor, it is the precursor to polyvinyl acetate, an important polymer in industry.-Production:...
and acrylamide
Acrylamide
Acrylamide is a chemical compound with the chemical formula C3H5NO. Its IUPAC name is prop-2-enamide. It is a white odourless crystalline solid, soluble in water, ethanol, ether, and chloroform. Acrylamide is incompatible with acids, bases, oxidizing agents, iron, and iron salts...
was examined.
The sulfur radical was found to be more reactive (6*108 vs. 1*107 mole-1.s-1) and less selective (selectivity ratio 76 vs 1200) than the carbon radical. In this case the effect can be explained by extending the Bell–Evans–Polanyi principle
Bell–Evans–Polanyi principle
In physical chemistry, the Bell–Evans–Polanyi principle observes that in a series of similar homolytic atom transfer reactions of the general type, there is often a closely linear relationship between the activation energy and the reaction enthalpy...
with a factor
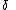 accounting for transfer of charge from the reactants to the transition state
accounting for transfer of charge from the reactants to the transition stateTransition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
of the reaction which can be calculated in silico
In silico
In silico is an expression used to mean "performed on computer or via computer simulation." The phrase was coined in 1989 as an analogy to the Latin phrases in vivo and in vitro which are commonly used in biology and refer to experiments done in living organisms and outside of living organisms,...
:
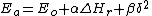
with
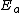 the activation energy
the activation energyActivation energy
In chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
and
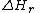 the reaction enthalpy
the reaction enthalpyEnthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
change. With the electrophilic
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
sulfur radical the charge transfer is largest with electron-rich alkenes such as acrylonitrile but the resulting reduction in activation energy (β is negative) is offset by a reduced enthalpy. With the nucleophilic
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
carbon radical on the other hand both enthalpy and polar effects have the same direction thus extending the activation energy range.

