
Danishefsky Taxol total synthesis
Encyclopedia
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
is an important third Taxol synthesis
Taxol total synthesis
Paclitaxel total synthesis in organic chemistry is a major ongoing research effort in the total synthesis of paclitaxel . This diterpenoid is an important drug in the treatment of cancer but also expensive because the compound is harvested from a scarce resource, namely the Pacific yew...
published by the group of Samuel Danishefsky in 1996
two years after the first two efforts described in the Holton Taxol total synthesis
Holton Taxol total synthesis
The Holton Taxol total synthesis, published by Robert A. Holton and his group at Florida State University in 1994 was the first total synthesis of Taxol ....
and the Nicolaou Taxol total synthesis
Nicolaou Taxol total synthesis
The Nicolaou Taxol total synthesis, published by K. C. Nicolaou and his group in 1994 concerns the total synthesis of Taxol. This organic synthesis was included in Nicolaou's book, 'Classics in Total Synthesis'....
. Combined they provide a good insight in the application of organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
in total synthesis
Total synthesis
In organic chemistry, a total synthesis is, in principle, the complete chemical synthesis of complex organic molecules from simpler pieces, usually without the aid of biological processes. In practice, these simpler pieces are commercially available in bulk and semi-bulk quantities, and are often...
.
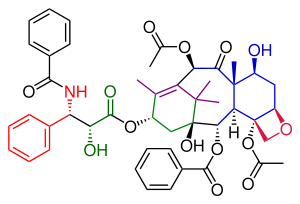
Danishefsky's route to Taxol has many similarities with that of Nicolaou. Both are examples of convergent synthesis
Convergent synthesis
In chemistry a convergent synthesis is a strategy that aims to improve the efficiency of multi-step chemical synthesis, most often in organic synthesis...
with a coupling of the A and the C ring from two precursors. The main characteristic of the Danishefsky variant is the completion of the oxetane
Oxetane
Oxetane, or 1,3-propylene oxide, is an heterocyclic organic compound with the molecular formula C3H6O, having a four-membered ring with three carbon atoms and one oxygen atom....
D ring onto the cyclohexanol
Cyclohexanol
Cyclohexanol is the organic compound with the formula 5CHOH. The molecule is related to cyclohexane ring by replacement of one hydrogen atom by a hydroxyl group. This compound exists as a deliquescent colorless solid, which, when very pure, melts near room temperature...
C ring prior to the construction of the 8-membered B ring. The most prominent starting material is the (+) enantiomer
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
of the Wieland-Miescher ketone
Wieland-Miescher ketone
The Wieland–Miescher ketone is a racemic bicyclic diketone and is a versatile synthon which has so far been employed in the total synthesis of more than 50 natural products, predominantly sesquiterpenoids, diterpenes and steroids possessing possible biological properties including anticancer,...
. This compound is commercially available as a single enantiomer
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
and the single chiral group present in this molecule is able to drive the entire sequence of organic reactions to a single optically active Taxol endproduct. The final step, the tail addition is identical to that of Nicolaou and is based on Ojima chemistry
Ojima lactam
The Ojima lactam is an organic compound of some importance in the commercial production of Taxol. This lactam was first synthesized by Iwao Ojima . The organic synthesis is an illustration of asymmetric synthesis via a chiral auxiliary....
.
In terms of raw material shopping, this taxol molecule consists of the aforementioned Wieland-Miescher ketone
Wieland-Miescher ketone
The Wieland–Miescher ketone is a racemic bicyclic diketone and is a versatile synthon which has so far been employed in the total synthesis of more than 50 natural products, predominantly sesquiterpenoids, diterpenes and steroids possessing possible biological properties including anticancer,...
, 2-methyl-3-pentanone, lithium aluminium hydride
Lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
, osmium tetroxide, phenyllithium
Phenyllithium
Phenyllithium is an organometallic agent with the empirical formula C6H5Li. It is most commonly used as a metalating agent in organic syntheses and a substitute for Grignard reagents for introducing phenyl groups in organic syntheses...
, pyridinium chlorochromate
Pyridinium chlorochromate
Pyridinium chlorochromate is a reddish orange solid reagent used to oxidize primary alcohols to aldehydes and secondary alcohols to ketones. Pyridinium chlorochromate, or PCC, will not fully oxidize a primary alcohol to the carboxylic acid as does the Jones reagent. A disadvantage to using PCC is...
, the Corey-Chaykovsky reagent and acryloyl chloride
Acryloyl chloride
Acryloyl chloride, also known as 2-propenoyl chloride or acrylic acid chloride, is a clear, light yellow, flammable liquid with an acrid smell. It belongs to the acid chlorides group of compounds and is therefore a derivative of acrylic acid.-Preparation:...
. Key chemical transformations are the Johnson-Corey-Chaykovsky reaction
Johnson-Corey-Chaykovsky reaction
The Johnson–Corey–Chaykovsky reaction is a chemical reaction used in organic chemistry for the synthesis of epoxides, aziridines, and cyclopropanes. It was discovered in 1961 by A. William Johnson and developed significantly by E.J. Corey and Michael Chaykovsky...
and the Heck reaction
Heck reaction
The Heck reaction is the chemical reaction of an unsaturated halide with an alkene and a base and palladium catalyst to form a substituted alkene. Together with the other palladium-catalyzed cross-coupling reactions, this reaction is of great importance, as it allows one to do substitution...
.
Retrosynthesis
TaxolPaclitaxel
Paclitaxel is a mitotic inhibitor used in cancer chemotherapy. It was discovered in a U.S. National Cancer Institute program at the Research Triangle Institute in 1967 when Monroe E. Wall and Mansukh C. Wani isolated it from the bark of the Pacific yew tree, Taxus brevifolia and named it taxol...
resulted from the tail addition of the Ojima lactam
Ojima lactam
The Ojima lactam is an organic compound of some importance in the commercial production of Taxol. This lactam was first synthesized by Iwao Ojima . The organic synthesis is an illustration of asymmetric synthesis via a chiral auxiliary....
to alcohol 51, which is baccatin III (the original target molecule of the Danishefsky synthesis). Alcohol 51 was derived from the allylic oxidation
Organoselenium chemistry
Organoselenium compounds are chemical compounds containing carbon-to-selenium chemical bonds. Organoselenium chemistry is the corresponding science exploring their properties and reactivity. Selenium belongs with oxygen and sulfur to the group 16 elements and similarities in chemistry are to be...
of α-acylketone 49. Compound 49 was ultimately derived from the Heck reaction
Heck reaction
The Heck reaction is the chemical reaction of an unsaturated halide with an alkene and a base and palladium catalyst to form a substituted alkene. Together with the other palladium-catalyzed cross-coupling reactions, this reaction is of great importance, as it allows one to do substitution...
of enol
Enol
Enols are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated anions of enols are called enolates...
triflate 38, which was used to close the B-ring. Enol triflate 38 resulted from a rearrangement of compound 31 after protection of its hydroxyl group. Compound 31 was derived from the connection of the A and C rings with aldehyde 21 combining with the vinyl
Vinyl
A vinyl compound is any organic compound that contains a vinyl group ,which are derivatives of ethene, CH2=CH2, with one hydrogen atom replaced with some other group...
lithium reagent derived from cyanohydrin
Cyanohydrin
A cyanohydrin is a functional group found in organic compounds. Cyanohydrins have the formula R2CCN, where R is H, alkyl, or aryl. Cyanohydrins are industrially important precursors to carboxylic acids and some amino acids...
29. Cyanohydrin 29 originated as the ethyl isopropyl ketone (22). Aldehyde 21 was obtained from compound 17, which was the product of the opening of ketal 12. Ketal 12 was ultimately derived from the Wieland-Miescher ketone
Wieland-Miescher ketone
The Wieland–Miescher ketone is a racemic bicyclic diketone and is a versatile synthon which has so far been employed in the total synthesis of more than 50 natural products, predominantly sesquiterpenoids, diterpenes and steroids possessing possible biological properties including anticancer,...
(1).
 |
| Retrosynthesis |
|---|
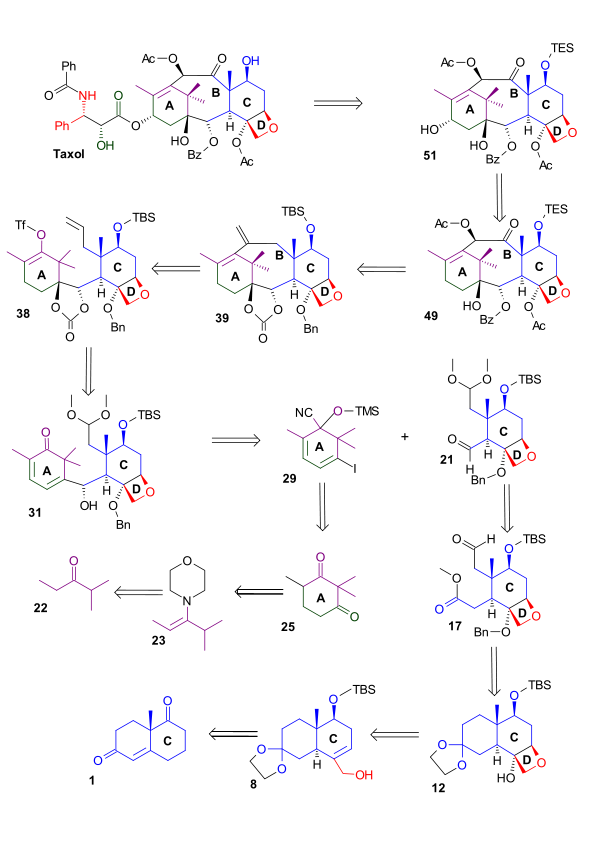
D Ring synthesis
Scheme 1 shows the synthesis of the oxetane D ring from the C ring starting from the (+) enantiomer of the Wieland-Miescher ketoneWieland-Miescher ketone
The Wieland–Miescher ketone is a racemic bicyclic diketone and is a versatile synthon which has so far been employed in the total synthesis of more than 50 natural products, predominantly sesquiterpenoids, diterpenes and steroids possessing possible biological properties including anticancer,...
(1). Reduction of this diketone with sodium borohydride
Sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate, is an inorganic compound with the formula NaBH4. This white solid, usually encountered as a powder, is a versatile reducing agent that finds wide application in chemistry, both in the laboratory and on a technical scale. Large amounts are...
provided unsaturated ketoalcohol 2, which was protected as an acetate. Formation of the ketal was accomponied by alkene rearrangement. The acetyl group was replaced by a tert-butyldimethylsilyl protecting group. Hydroboration followed by oxidation with hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
gave alcohol 5. The hydroxyl group was then oxidized to a carbonyl group giving ketone 6 by action of pyridinium dichromate. With all the sensitive functional groups protected, the methylene group required for the oxetane
Oxetane
Oxetane, or 1,3-propylene oxide, is an heterocyclic organic compound with the molecular formula C3H6O, having a four-membered ring with three carbon atoms and one oxygen atom....
ring D was then provided by the Corey-Chaykovsky reagent, which converted the carbonyl group to an epoxide (7). Treatment of this epoxide with aluminium isopropoxide
Aluminium isopropoxide
Aluminium isopropoxide is the chemical compound usually described with the formula Al3, where i-Pr is the isopropyl group . This colourless solid is a useful reagent in organic synthesis...
gave allylic alcohol 8. Two more hydroxyl groups were added by oxidation of the newly formed double bond with a catalytic amount of osmium tetroxide in the presence of N-methylmorpholine N-oxide
N-Methylmorpholine N-oxide
N-Methylmorpholine-N-oxide, NMO or NMMO is an organic compound. This heterocyclic amine oxide and morpholine derivative is used in organic chemistry as a co-oxidant and sacrificial catalyst in oxidation reactions for instance in osmium tetroxide oxidations and the Sharpless asymmetric...
. This reaction lacked stereospecificity and the yield of triol 9 with the correct stereochemistry
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
was therefore reduced. The primary alcohol was protected as a silyl ether
Silyl ether
Silyl ethers are a group of chemical compounds which contain a silicon atom covalently bonded to an alkoxy group. The general structure is R1R2R3Si−O−R4 where R4 is an alkyl group or an aryl group. Silyl ethers are usually used as protecting groups for alcohols in organic synthesis...
and the secondary alcohol was activated as a triflate
Triflate
Trifluoromethanesulfonate, also known by the trivial name triflate, is a functional group with the formula CF3SO3-. The triflate group is often represented by -OTf, as opposed to -Tf...
(11). Heating this trimethylsilyl protected triflate in refluxing ethlyene glycol closed the ring to give oxetane 12.
 |
| Scheme 1 |
|---|
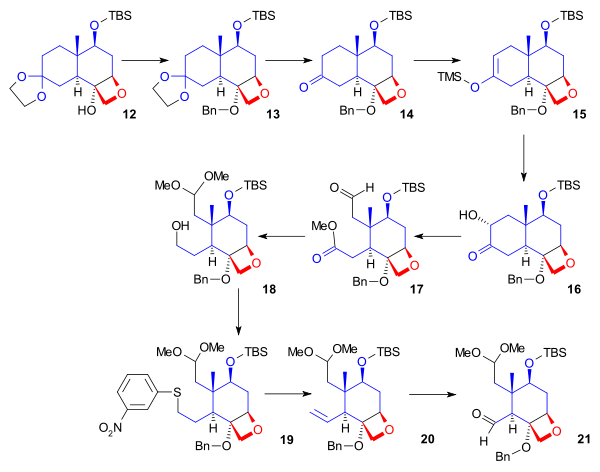
Preparation for AB Ring synthesis
In the next phase (Scheme 2), starting from ketal 12, the cyclohexane ring was cleaved to provide two anchoring points for fusion with the A ring. Alcohol 12 was protected by a benzyl group. The acetonide protecting groupProtecting group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group in order to obtain chemoselectivity in a subsequent chemical reaction...
was removed from the ketone. Ketone 14 was converted to silyl enol ether
Silyl enol ether
Silyl enol ethers in organic chemistry are a class of organic compounds that share a common functional group composed of an enolate bonded through its oxygen terminus to an organosilicon group....
15 by reaction with trimethylsilyl triflate, and a modified Rubottom oxidation
Rubottom oxidation
The Rubottom oxidation is the chemical reaction of enolsilanes with m-chloroperoxybenzoic acid to give silyl-protected α-hydroxy ketones.-Reaction mechanism:...
using 3,3-dimethyldioxirane
Dimethyldioxirane
Dimethyldioxirane is a dioxirane derived from acetone. It is the most commonly used dioxirane in organic synthesis.-Synthesis:DMDO is not commercially available because of its instability...
followed by a treatment with camphorsulfonic acid
Camphorsulfonic acid
Camphorsulfonic acid, sometimes abbreviated CSA or 10-CSA is a organosulfur compound. Like typical sulfonic acids, it is a relatively strong acid that exists as a colourless solid that is soluble in organic solvents....
introduced a hydroxyl group alpha to the ketone. Ring opening by oxidative cleavage with lead tetraacetate in methanol gave compound 17. In the next step, the aldehyde was protected as a dimethyl acetal, and the ester was reduced to give primary alcohol 18. The hydroxyl group was converted in a Grieco elimination
Grieco elimination
The Grieco elimination is an organic reaction describing the elimination reaction of an aliphatic primary alcohol through a selenide to a terminal alkene ....
to the selenide
Selenide
A selenide is a chemical compound in which selenium serves as an anion with oxidation number of −2 , much as sulfur does in a sulfide. The chemistry of the selenides and sulfides are similar....
(19), which on oxidation with hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
gave alkene 20. Ozonolysis
Ozonolysis
Ozonolysis is the cleavage of an alkene or alkyne with ozone to form organic compounds in which the multiple carbon–carbon bond has been replaced by a double bond to oxygen...
with ozone
Ozone
Ozone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
and triphenylphosphine
Triphenylphosphine
Triphenylphosphine is a common organophosphorus compound with the formula P3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature...
provided aldehyde 21.
 |
| Scheme 2 |
|---|
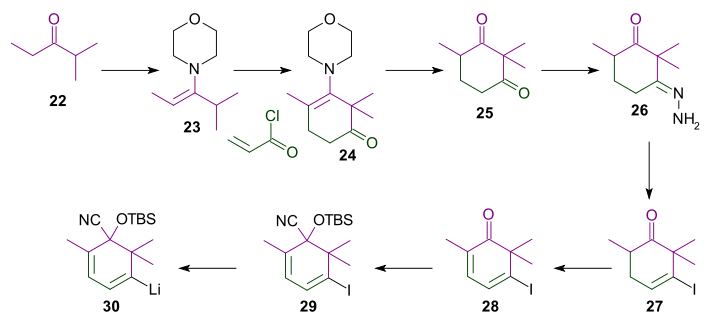
AB Ring synthesis
For this synthesis (Scheme 3) the morpholine enamine of ethyl isopropyl ketone was reacted with acryloyl chlorideAcryloyl chloride
Acryloyl chloride, also known as 2-propenoyl chloride or acrylic acid chloride, is a clear, light yellow, flammable liquid with an acrid smell. It belongs to the acid chlorides group of compounds and is therefore a derivative of acrylic acid.-Preparation:...
in a combined nucleophilic conjugate addition
Nucleophilic conjugate addition
Nucleophilic conjugate addition is a type of organic reaction. Ordinary nucleophilic additions or 1,2-nucleophilic additions deal mostly with additions to carbonyl compounds. Simple alkene compounds do not show 1,2 reactivity due to lack of polarity, unless the alkene is activated with special...
and nucleophilic acyl substitution
Nucleophilic acyl substitution
Nucleophilic acyl substitution describes the substitution reaction involving nucleophiles and acyl compounds. Acyl compounds are carboxylic acid derivatives including esters, amides and acid halides...
to give after hydrolysis diketone 25. Reaction with hydrazine
Hydrazine
Hydrazine is an inorganic compound with the formula N2H4. It is a colourless flammable liquid with an ammonia-like odor. Hydrazine is highly toxic and dangerously unstable unless handled in solution. Approximately 260,000 tons are manufactured annually...
in triethylamine
Triethylamine
Triethylamine is the chemical compound with the formula N3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine, for which TEA is also a common abbreviation....
and ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
afforded hydrazone
Hydrazone
Hydrazones are a class of organic compounds with the structure R1R2C=NNH2. They are related to ketones and aldehydes by the replacement of the oxygen with the NNH2 functional group...
26. After an unusual hydrazone iodination
Hydrazone iodination
Hydrazone iodination is an organic reaction in which a hydrazone is converted into a vinyl iodide by reaction of iodine and a non-nucleophilic base such as DBU. First published by D. H. R...
that also involved iodination alpha to a carbonyl group and elimination of HI, fully conjugated vinyl iodide 28 was produced in an unexpected dehydrogenation
Dehydrogenation
Dehydrogenation is a chemical reaction that involves the elimination of hydrogen . It is the reverse process of hydrogenation. Dehydrogenation reactions may be either large scale industrial processes or smaller scale laboratory procedures....
. The ketone was converted into cyanohydrin
Cyanohydrin
A cyanohydrin is a functional group found in organic compounds. Cyanohydrins have the formula R2CCN, where R is H, alkyl, or aryl. Cyanohydrins are industrially important precursors to carboxylic acids and some amino acids...
29 with trimethylsilyl cyanide
Trimethylsilyl cyanide
Trimethylsilyl cyanide is the chemical compound with the formula 3SiCN. This volatile liquid consists of a cyanide group, that is CN, attached to a trimethylsilyl group. The molecule is used in organic synthesis as the equivalent of hydrogen cyanide...
, potassium cyanide
Potassium cyanide
Potassium cyanide is an inorganic compound with the formula KCN. This colorless crystalline compound, similar in appearance to sugar, is highly soluble in water. Most KCN is used in gold mining, organic synthesis, and electroplating. Smaller applications include jewelry for chemical gilding and...
and a crown ether
Crown ether
Crown ethers are cyclic chemical compounds that consist of a ring containing several ether groups. The most common crown ethers are oligomers of ethylene oxide, the repeating unit being ethyleneoxy, i.e., -CH2CH2O-. Important members of this series are the tetramer , the pentamer , and the hexamer...
.
 |
| Scheme 3 |
|---|
As shown in Scheme 4, the bottom part of the taxol B ring synthesis involved the reaction of ring C aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
group of 21. The ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
group was deprotected by action of tetra-n-butylammonium fluoride
Tetra-n-butylammonium fluoride
Tetra-n-butylammonium fluoride or TBAF is a quaternary ammonium salt with the chemical formula 4N+F-. It is commercially available as the trihydrate and as a solution in tetrahydrofuran....
, and the double bond was epoxidized with meta-chloroperoxybenzoic acid
Meta-Chloroperoxybenzoic acid
meta-Chloroperoxybenzoic acid is a peroxycarboxylic acid used widely as an oxidant in organic synthesis. mCPBA is often preferred to other peroxy acids because of its relative ease of handling...
to epoxide 32. This epoxide was then hydrogenated
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
with hydrogen over palladium on carbon
Palladium on carbon
Palladium on carbon, often referred to as Pd/C, is a form of palladium used for catalysis. It is usually used for catalytic hydrogenations in organic chemistry...
to give diol 33, which was protected in the next step as the cyclic carbonate ester
Carbonate ester
A carbonate ester is a functional group in organic chemistry consisting of a carbonyl group flanked by two alkoxy groups. The general structure of these carbonates is R1OOR2 and they are related to esters R1OR and ethers R1OR2 and also to the inorganic carbonates.Carbonate esters are used as...
(34).
L-Selectride
L-selectride
L-selectride is an organoborane. It is used in organic chemistry as a reducing agent, for example in the reduction of a ketone, as part of Overman's synthesis of strychnine....
reduction of enone 34 gave ketone 35. The ketone was converted into vinyl
Vinyl
A vinyl compound is any organic compound that contains a vinyl group ,which are derivatives of ethene, CH2=CH2, with one hydrogen atom replaced with some other group...
triflate
Triflate
Trifluoromethanesulfonate, also known by the trivial name triflate, is a functional group with the formula CF3SO3-. The triflate group is often represented by -OTf, as opposed to -Tf...
36 using phenyl triflimide and potassium hexamethyldisilazide in tetrahydrofuran
Tetrahydrofuran
Tetrahydrofuran is a colorless, water-miscible organic liquid with low viscosity at standard temperature and pressure. This heterocyclic compound has the chemical formula 4O. As one of the most polar ethers with a wide liquid range, it is a useful solvent. Its main use, however, is as a precursor...
at −78 °C. This vinyl triflate was one of the functional groups required for the Heck reaction
Heck reaction
The Heck reaction is the chemical reaction of an unsaturated halide with an alkene and a base and palladium catalyst to form a substituted alkene. Together with the other palladium-catalyzed cross-coupling reactions, this reaction is of great importance, as it allows one to do substitution...
. For the generation of the other reactive group the acetal
Acetal
An acetal is a molecule with two single-bonded oxygen atoms attached to the same carbon atom.Traditional usages distinguish ketals from acetals...
group was removed to give aldehyde 37 which was subsequently converted to the terminal alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
38 in a Wittig reaction
Wittig reaction
The Wittig reaction is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide to give an alkene and triphenylphosphine oxide....
involving methylenetriphenylphosphorane
Phosphorane
A phosphorane is a functional group in organophosphorus chemistry with pentavalent phosphorus. It has the general formula PR5. The parent hydride compound is the unstable molecule PH5...
. The intramolecular Heck reaction
Heck reaction
The Heck reaction is the chemical reaction of an unsaturated halide with an alkene and a base and palladium catalyst to form a substituted alkene. Together with the other palladium-catalyzed cross-coupling reactions, this reaction is of great importance, as it allows one to do substitution...
involved tetrakis(triphenylphosphine)palladium(0)
Tetrakis(triphenylphosphine)palladium(0)
Tetrakispalladium is the chemical compound Pd[P3]4, often abbreviated Pd4, or even PdP4. It is a bright yellow crystalline solid that becomes brown upon decomposition in air.-Structure and properties:...
and potassium carbonate
Potassium carbonate
Potassium carbonate is a white salt, soluble in water , which forms a strongly alkaline solution. It can be made as the product of potassium hydroxide's absorbent reaction with carbon dioxide. It is deliquescent, often appearing a damp or wet solid...
in acetonitrile
Acetonitrile
Acetonitrile is the chemical compound with formula . This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture...
at reflux
Reflux
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations...
to give diene 39 and to complete the formation of the B ring.
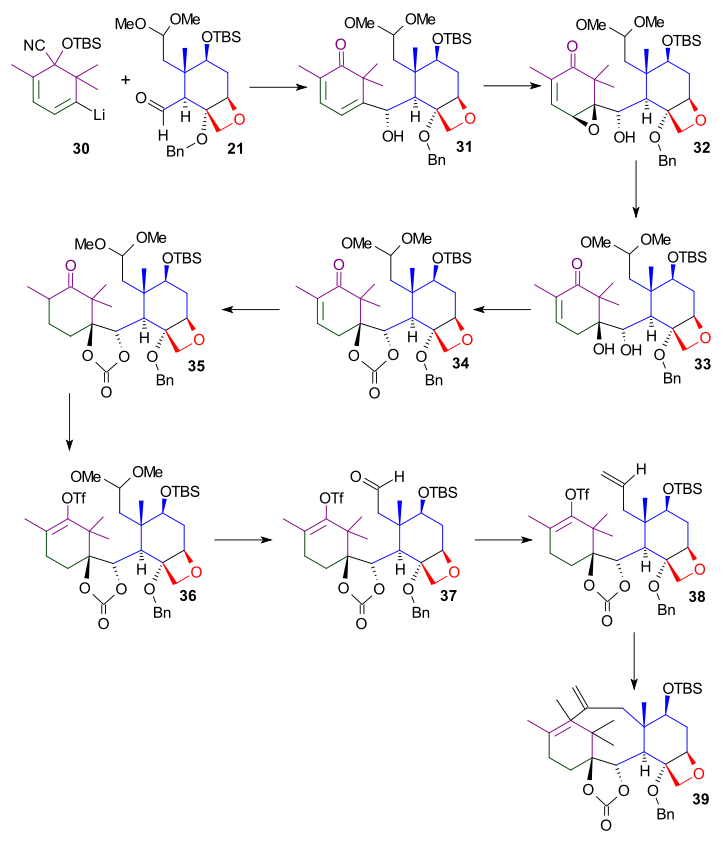 |
| Scheme 4 |
|---|
B Ring elaboration
The second part of the B ring synthesis (Scheme 5) was concerned with correct chemistry for the newly formed ethylene bridge connecting the A and C rings. After Scheme 4, this bridge contained an exocyclic methylene group, but in the ultimate taxol molecule this bridge is an α-acylketone. The required conversion was accomplished in the next 10 steps.The tert-butylsilyl protecting group in diene 39 was not compatible in later reactions and was replaced by a triethylsilyl
Silyl ether
Silyl ethers are a group of chemical compounds which contain a silicon atom covalently bonded to an alkoxy group. The general structure is R1R2R3Si−O−R4 where R4 is an alkyl group or an aryl group. Silyl ethers are usually used as protecting groups for alcohols in organic synthesis...
. Epoxidation of diene 40 with meta-chloroperoxybenzoic acid gave the oxirane ring. This served solely as a protecting group in preparation for modifications of the exocyclic alkene. In the next two steps, the benzyl protecting group in compound 41 was replaced by an acetyl group. Carbonate ester
Carbonate ester
A carbonate ester is a functional group in organic chemistry consisting of a carbonyl group flanked by two alkoxy groups. The general structure of these carbonates is R1OOR2 and they are related to esters R1OR and ethers R1OR2 and also to the inorganic carbonates.Carbonate esters are used as...
43 was opened by reaction with phenyllithium
Phenyllithium
Phenyllithium is an organometallic agent with the empirical formula C6H5Li. It is most commonly used as a metalating agent in organic syntheses and a substitute for Grignard reagents for introducing phenyl groups in organic syntheses...
to give alcohol 44. The cleavage of the exocyclic double bond was difficult and accomplished only with forcing conditions (19 equivalents of osmium textroxide, 105 °C, 24 hours) by the putative osmate ester (45). Subsequent oxidative cleavage with lead tetraacetate gave ketone 46. The epoxide protecting group was removed with samarium (II) iodide to give α-ß-unsaturated ketone 47. The enolate was formed by the reaction of ketone 47 with potassium tert-butoxide
Potassium tert-butoxide
Potassium tert-butoxide is the chemical compound with the formula 3COK. This colourless solid is a strong base useful in organic synthesis. It exists as a tetrameric cubane-like cluster...
, and subsequent reaction with phenylseleninic anhydride followed by acylation gave α-acylketone 49.
 |
| Scheme 5 |
|---|
Tail addition
The tail addition step in this synthesis (Scheme 6) was identical to that in the Nicolaou tail addition and was based on Oijma chemistryOjima lactam
The Ojima lactam is an organic compound of some importance in the commercial production of Taxol. This lactam was first synthesized by Iwao Ojima . The organic synthesis is an illustration of asymmetric synthesis via a chiral auxiliary....
. The A ring was functionalized with a hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
group through pyridinium chlorochromate
Pyridinium chlorochromate
Pyridinium chlorochromate is a reddish orange solid reagent used to oxidize primary alcohols to aldehydes and secondary alcohols to ketones. Pyridinium chlorochromate, or PCC, will not fully oxidize a primary alcohol to the carboxylic acid as does the Jones reagent. A disadvantage to using PCC is...
oxidation of α-acylketone 49 to form ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
50. Subsequent reduction using sodium borohydride
Sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate, is an inorganic compound with the formula NaBH4. This white solid, usually encountered as a powder, is a versatile reducing agent that finds wide application in chemistry, both in the laboratory and on a technical scale. Large amounts are...
produced alcohol 51 . Reaction of this alcohol with the Ojima lactam
Ojima lactam
The Ojima lactam is an organic compound of some importance in the commercial production of Taxol. This lactam was first synthesized by Iwao Ojima . The organic synthesis is an illustration of asymmetric synthesis via a chiral auxiliary....
52 and a concluding silyl deprotection step at two triethyl silyl
Silyl ether
Silyl ethers are a group of chemical compounds which contain a silicon atom covalently bonded to an alkoxy group. The general structure is R1R2R3Si−O−R4 where R4 is an alkyl group or an aryl group. Silyl ethers are usually used as protecting groups for alcohols in organic synthesis...
positions in compound 53 gave Taxol.
 |
| Scheme 6 |
|---|
Ac (acetate)
Protection: acetic anhydrideAcetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula 2O. Commonly abbreviated Ac2O, it is the simplest isolatable acid anhydride and is a widely used reagent in organic synthesis...
, 4-(dimethylamino)pyridine and pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
.
Deprotection: sodium ethoxide
Sodium ethoxide
Sodium ethoxide is an alkoxide salt with the chemical formula C2H5ONa.-Preparation:It is commercially available as a white solid, or as a solution in ethanol. It is easily prepared in the laboratory by reacting sodium metal with ethanol:...
, ethanol
The hydroxyl group in alcohol 3 (Scheme 1) was protected as an acetate during the subsequent alkene rearrangement. The acetate was removed by a tert-butyldimethylsilyl protecting group (ketone 6).
Acetonide
Protection: ethylene glycolEthylene glycol
Ethylene glycol is an organic compound widely used as an automotive antifreeze and a precursor to polymers. In its pure form, it is an odorless, colorless, syrupy, sweet-tasting liquid...
, naphthalenesulfonic acid
Deprotection: P-toluenesulfonic acid
P-Toluenesulfonic acid
p-Toluenesulfonic acid or tosylic acid is an organic compound with the formula CH3C6H4SO3H. It is a white solid that is soluble in water, alcohols, and other polar organic solvents. The 4-CH3C6H4SO2- group is known as the Tosyl group and is often abbreviated as Ts or Tos...
, acetone, and water.
Ketone 3 (Scheme 1) was protected using an acetonide group, which was removed after the closure of ring D (ketone 14, Scheme 2).
Bn (benzyl)
Protection: benzyl chlorideBenzyl chloride
Benzyl chloride, or α-chlorotoluene, is an organic compound with the formula C6H5CH2Cl. This colourless liquid is a reactive organochlorine compound that is a widely used chemical building block.-Preparation:...
, pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
Deprotection: acetic anhydride
Acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula 2O. Commonly abbreviated Ac2O, it is the simplest isolatable acid anhydride and is a widely used reagent in organic synthesis...
, 4-(dimethylamino)pyridine, and pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
The hydroxyl group in ketal 12 (Scheme 2) was protected as a benzyl ether, which was replaced much later in the synthesis (alcohol 42, Scheme 5)
Carbonate Ester (cyclic)
Protection: carbonyl diimidazole, sodium hydrideSodium hydride
Sodium hydride is the chemical compound with the empirical formula NaH. It is primarily used as a strong base in organic synthesis. NaH is representative of the saline hydrides, meaning it is a salt-like hydride, composed of Na+ and H− ions, in contrast to the more molecular hydrides such as...
, dimethylformamide
Dimethylformamide
Dimethylformamide is an organic compound with the formula 2NCH. Commonly abbreviated as DMF , this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions...
Deprotection: phenyllithium
Phenyllithium
Phenyllithium is an organometallic agent with the empirical formula C6H5Li. It is most commonly used as a metalating agent in organic syntheses and a substitute for Grignard reagents for introducing phenyl groups in organic syntheses...
Diol 33 was protected as a cyclic carbonate ester (Scheme 4). Treatment of the carbonate ester with phenyllithium deprotected one hydroxyl group and left a benzoyl group needed for Taxol on the other oxygen (alcohol 44, Scheme 5).
Dimethyl Acetal
Protection: methanol, collidine p-toluenesulfonateCollidine p-toluenesulfonate
Collidinium p-toluenesulfonate or CTPS is a salt between p-toluenesulfonic acid and collidine . It is used as a mild glycosylation catalyst in chemistry....
Deprotection: pyridinium tosylate
The carbonyl group in aldehyde 17 of Scheme 2 was protected as the dimethyl acetal in order to allow the addition of the A ring to the C ring (Scheme 4). The aldehyde is later deprotected using pyridinium tosylate later on in Scheme 4.
Epoxide
Protection: meta-chloroperoxybenzoic acidMeta-Chloroperoxybenzoic acid
meta-Chloroperoxybenzoic acid is a peroxycarboxylic acid used widely as an oxidant in organic synthesis. mCPBA is often preferred to other peroxy acids because of its relative ease of handling...
Deprotection: samarium(II) iodide
Samarium(II) iodide
Samarium iodide is a green solid composed of samarium and iodine, with a melting point of 520 °C where the samarium atom has a coordination number of seven in a capped octahedral configuration...
, acetic anhydride
Acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula 2O. Commonly abbreviated Ac2O, it is the simplest isolatable acid anhydride and is a widely used reagent in organic synthesis...
, tetrahydrofuran
ThF
Follicular B helper T cells , are antigen-experienced CD4+ T cells found in the B cell follicles of secondary lymphoid organs such as lymph nodes, spleens and Peyer's patches, and are identified by their constitutive expression of the B cell follicle homing receptor CXCR5...
One of the double-bonds of diene 39 (Scheme 5) was protected as an epoxide in preparation for modifications of the other alkene. The epoxide was present for 4 steps before being removed with samarium(II) oxide.
TBS (tert-butyldimethylsilyl)
Protection: tertbutyldimethylsilyl chloride, lutidineDeprotection: Tetra-n-butylammonium fluoride
Tetra-n-butylammonium fluoride
Tetra-n-butylammonium fluoride or TBAF is a quaternary ammonium salt with the chemical formula 4N+F-. It is commercially available as the trihydrate and as a solution in tetrahydrofuran....
, tetrahydrofuran
ThF
Follicular B helper T cells , are antigen-experienced CD4+ T cells found in the B cell follicles of secondary lymphoid organs such as lymph nodes, spleens and Peyer's patches, and are identified by their constitutive expression of the B cell follicle homing receptor CXCR5...
The acetate protecting group required for the acetylation of enone 3 (Scheme 1) was replaced by the more robust tert-butyldimethylsilyl protecting group. This silyl group was removed much later, in order to have a more easily-removed protecting group present for the final steps of the Taxol synthesis (compound 40, Scheme 5).
TES (triethylsilyl) [1]
Protection: triethylsilyl triflate, triethylamineTriethylamine
Triethylamine is the chemical compound with the formula N3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine, for which TEA is also a common abbreviation....
, and dichloromethane
Dichloromethane
Dichloromethane is an organic compound with the formula CH2Cl2. This colorless, volatile liquid with a moderately sweet aroma is widely used as a solvent. Although it is not miscible with water, it is miscible with many organic solvents...
.
Deprotection: hydrogen fluoride, pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
, and acetonitrile
Acetonitrile
Acetonitrile is the chemical compound with formula . This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture...
A triethylsilyl group replaced a tert-butyl silyl protecting group late in the synthesis (compound 40, Scheme 5). The TES group is later removed in the concluding silyl deprotection step that gives the final Taxol compound.
TES (triethylsilyl) [2]
Protection: See: Ojima lactamOjima lactam
The Ojima lactam is an organic compound of some importance in the commercial production of Taxol. This lactam was first synthesized by Iwao Ojima . The organic synthesis is an illustration of asymmetric synthesis via a chiral auxiliary....
Deprotection: hydrogen fluoride, pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
, and acetonitrile
Acetonitrile
Acetonitrile is the chemical compound with formula . This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture...
The TES protecting group that was present in the Ojima lactam is removed in the concluding silyl deprotection step of the Taxol total synthesis.
TMS (trimethylsilyl)
Protection: trimethylsilyl cyanideTrimethylsilyl cyanide
Trimethylsilyl cyanide is the chemical compound with the formula 3SiCN. This volatile liquid consists of a cyanide group, that is CN, attached to a trimethylsilyl group. The molecule is used in organic synthesis as the equivalent of hydrogen cyanide...
, potassium cyanide
Potassium cyanide
Potassium cyanide is an inorganic compound with the formula KCN. This colorless crystalline compound, similar in appearance to sugar, is highly soluble in water. Most KCN is used in gold mining, organic synthesis, and electroplating. Smaller applications include jewelry for chemical gilding and...
, and a crown ether
Crown ether
Crown ethers are cyclic chemical compounds that consist of a ring containing several ether groups. The most common crown ethers are oligomers of ethylene oxide, the repeating unit being ethyleneoxy, i.e., -CH2CH2O-. Important members of this series are the tetramer , the pentamer , and the hexamer...
Deprotection: tetra-n-butylammonium fluoride displacement
The ketone of diene 28 (Scheme 3) was protected by the trimethylsilyl group, and was removed when the vinyl lithium 30 group of A ring was added to the C ring aldehyde group in 21 (Scheme 4).
See also
- Paclitaxel total synthesis
- Holton Taxol total synthesisHolton Taxol total synthesisThe Holton Taxol total synthesis, published by Robert A. Holton and his group at Florida State University in 1994 was the first total synthesis of Taxol ....
- Kuwajima Taxol total synthesisKuwajima Taxol total synthesisThe Kuwajima Taxol total synthesis by the group of Isao Kuwajima of the Tokyo Institute of Technology is one of several efforts in taxol total synthesis published in the 1990s...
- Mukaiyama Taxol total synthesisMukaiyama Taxol total synthesisThe Mukaiyama taxol total synthesis published by the group of Teruaki Mukaiyama of the Tokyo University of Science between 1997 and 1999 was the 6th successful taxol total synthesis. The total synthesis of Taxol is considered a hallmark in organic synthesis....
- Nicolaou Taxol total synthesisNicolaou Taxol total synthesisThe Nicolaou Taxol total synthesis, published by K. C. Nicolaou and his group in 1994 concerns the total synthesis of Taxol. This organic synthesis was included in Nicolaou's book, 'Classics in Total Synthesis'....
- Wender Taxol total synthesisWender Taxol total synthesisThe Wender Taxol total synthesis in organic chemistry describes a Taxol total synthesis by the group of Paul A. Wender at Stanford University published in 1997. This synthesis has much in common with the Holton Taxol total synthesis in that it is a linear synthesis starting from a naturally...

