Rubottom oxidation
Encyclopedia
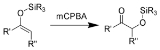
The Rubottom oxidation is the chemical reaction
of enol
silane
s with m-chloroperoxybenzoic acid to give silyl-protected
α-hydroxy ketones.
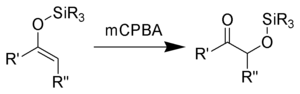
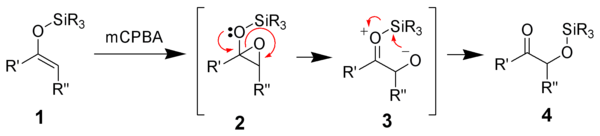
of the enolsilane (1) with m-chloroperoxybenzoic acid initially gives an epoxysilane (2). Rearrangement through a zwitterionic intermediate (3) gives the desired α-hydroxy ketone (4).
) or reversible (alkoxide
) base for deprotonation, respectively.
Alternative oxidants include hypervalent iodine reagents such as iodobenzene diacetate, molecular oxygen, metal oxides (most notably osmium tetroxide), and N-sulfonyloxaziridines.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
of enol
Enol
Enols are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated anions of enols are called enolates...
silane
Silane
Silane is a toxic, extremely flammable chemical compound with chemical formula SiH4. In 1857, the German chemists and Friedrich Woehler discovered silane among the products formed by the action of hydrochloric acid on aluminum silicide, which they had previously prepared...
s with m-chloroperoxybenzoic acid to give silyl-protected
Protecting group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group in order to obtain chemoselectivity in a subsequent chemical reaction...
α-hydroxy ketones.

Reaction mechanism
OxidationRedox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
of the enolsilane (1) with m-chloroperoxybenzoic acid initially gives an epoxysilane (2). Rearrangement through a zwitterionic intermediate (3) gives the desired α-hydroxy ketone (4).

Scope and Limitations
Although silyl enol ethers are the most common substrates for the Rubottom oxidation, peracids can be used to oxidize a variety of stabilized anions in addition to silyl enol ethers. Ketone and ester enolates, α-nitrile anions, α-imino anions, and silyl ketene acetals are all within the scope of the reaction. Site selective oxidation of these substrates depends on the selective deprotonation of the parent neutral compounds; selectivity is typically achieved by enforcing kinetic or thermodynamic control through the use of an irreversible (lithium diisopropylamideLithium diisopropylamide
Lithium diisopropylamide is the chemical compound with the formula [2CH]2NLi. Generally abbreviated LDA, it is a strong base used in organic chemistry for the deprotonation of weakly acidic compounds. The reagent has been widely accepted because it is soluble in non-polar organic solvents and it...
) or reversible (alkoxide
Alkoxide
An alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They can be written as RO−, where R is the organic substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands...
) base for deprotonation, respectively.
Alternative oxidants include hypervalent iodine reagents such as iodobenzene diacetate, molecular oxygen, metal oxides (most notably osmium tetroxide), and N-sulfonyloxaziridines.

