
Enol
Encyclopedia
Enols are alkene
s with a hydroxyl group affixed to one of the carbon atoms composing the double bond
. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated
anions of enols are called enolates. A reductone is a compound that has an enediol structure with an adjacent carbonyl-group.
The C=C double bond with adjacent alcohol gives enols and enediols their chemical characteristics, by which they present keto-enol tautomerism
. In keto-enol tautomerism, enols interconvert with ketone
s or aldehyde
s.
The words enol and alkenol are portmanteaus of the words "alkene
" (or just -ene
, the suffix given to C=C double bonded alkenes) and "alcohol
" (which represents the enol's hydroxyl group).
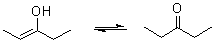 Enols interconvert with carbonyl compounds that have an α-hydrogen, like ketones and aldehydes. The compound is deprotonated
Enols interconvert with carbonyl compounds that have an α-hydrogen, like ketones and aldehydes. The compound is deprotonated
on one side and protonated
on another side, whereas a single bond and a double bond are exchanged. This is called keto-enol tautomerism
.
The enol form is usually unstable, does not survive long, and changes into the keto (ketone). This is because oxygen
is more electronegative than carbon
and thus forms stronger
bonds
.
ing and possibly to an easy internal proton transfer.
 Thus, at equilibrium, over 99% of propanedial (OHCCH2CHO) molecules exist as the mono-enol. The percentage is lower for 1,3-aldehyde ketones and diketones (acetylacetone
Thus, at equilibrium, over 99% of propanedial (OHCCH2CHO) molecules exist as the mono-enol. The percentage is lower for 1,3-aldehyde ketones and diketones (acetylacetone
, for example, 80 % enol form).
. Enolates can exist in quantitative amounts in strictly Brønsted acid free conditions, since they are generally very basic. In enolates the anionic charge is delocalized over the oxygen and the carbon
. Enolates are somewhat stabilized by this delocalization of the charge over three atoms. In older descriptions of bonding, particularly valence bond theory
this was explained by a phenomenon known as resonance
.
 Later theories of chemical bonding, particularly the theory of Molecular Orbitals
Later theories of chemical bonding, particularly the theory of Molecular Orbitals
do away with the twin resonant structures, where electrons are assumed to be attached either to one or to two atoms only. Instead it is assumed that bonding electrons can very well be shared by three (or more) atoms. This is hard to picture when electrons are imagined as particles only. However, duality
stipulates that they can also be seen as waves creating a standing wave pattern around all the atoms. Enolate presents an interesting and fairly simple example of the usefulness of this approach, because one can limit the discussion of the delocalization in terms of the number of contributing atomic orbitals to only three in this case: one pz orbital on each of the atoms C-C-O and leave the underlying skeleton of sigma bonds out of the picture.
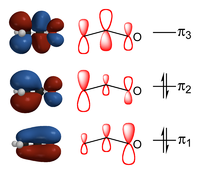
When combining these three pz-orbitals, that were assumed involved in the "resonance" above, three molecular orbital
s can be formed. The one lowest in energy does not possess any vertical nodal planes separating the atoms. The energy of this orbital is lower than the energies of the atomic orbitals that it originates from. It therefore constitutes a bonding orbital as its formation stabilizes the electrons residing in it. Its bonding has π-symmetry because it inherits the horizontal nodal plane form the p-orbitals it is composed of. In the figure this is shown by the blue and red colors above and below the plane of the nuclei, indicating the opposite phase of the wave on either side of the nodal plane. The second lowest orbital is essentially non-bonding in nature; its energy is not very different from the energies of the parent atomic orbitals. It has one additional nodal plane that resides vertically (not quite) on the central atom. The result is the alternating four red and blue color regions in the figure. The last MO is higher in energy than the parent atomic orbitals and therefore antibonding in nature. It has two additional vertical nodes, leading to six red or blue regions.
Fortunately, the top antibonding orbital is empty in the ground state on the ion, because the number of electrons involved in the "resonance" is only four. This avoids having to pay the energetic price of destabilization that its elevated energy implies. The fact that there are only four electrons means that only the bonding and the non-bonding MO (π1 and π2) are filled. Thus there is one bonding pair spread over two bonding regions, adding half a bond order to the sigma bond already present between the atoms. In this model we would therefore expect two fairly equivalent bonds of bond order 1½ each, rather that one bond with order 2 and one bond with order one.
In this description only a single electronic structure is present, rather than two, in which the negative charge is distributed over the three atoms, albeit not entirely evenly. The π1 orbital puts much of the charge on the oxygen atom, the π2 one on the terminal carbon atom, leaving the middle atom with less of the negative charge.
) the "kinetic" proton may be removed. The "kinetic" proton is the one which is sterically
most accessible. Under thermodynamic conditions (higher temperatures, weak base, and protic solvent) equilibrium is established between the ketone and the two possible enolates, the enolate favoured is termed the "thermodynamic" enolate and is favoured because of its lower energy level than the other possible enolate. Thus, by choosing the optimal conditions to generate an enolate, one can increase the yield of the desired product while minimizing formation of undesired products.
s in the Lobry-de Bruyn-van Ekenstein transformation
.
, resulting from the tautomerism with the adjacent carbonyl. Therefore, the chemical equilibrium
produces mainly the enediol form rather than the keto form. Reductones are strong reducing agents, thus efficacious antioxidant
s, and fairly strong acids.
Examples of reductones are tartronaldehyde, reductic acid and ascorbic acid.
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s with a hydroxyl group affixed to one of the carbon atoms composing the double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated
Deprotonation
Deprotonation is the removal of a proton from a molecule, forming the conjugate base.The relative ability of a molecule to give up a proton is measured by its pKa value. A low pKa value indicates that the compound is acidic and will easily give up its proton to a base...
anions of enols are called enolates. A reductone is a compound that has an enediol structure with an adjacent carbonyl-group.
The C=C double bond with adjacent alcohol gives enols and enediols their chemical characteristics, by which they present keto-enol tautomerism
Keto-enol tautomerism
In organic chemistry, keto-enol tautomerism refers to a chemical equilibrium between a keto form and an enol . The enol and keto forms are said to be tautomers of each other...
. In keto-enol tautomerism, enols interconvert with ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s or aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s.
The words enol and alkenol are portmanteaus of the words "alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
" (or just -ene
-ene
The suffix -ene is used in organic chemistry to form names of organic compounds where the -C=C- group has been attributed the highest priority according to the rules of organic nomenclature. Sometimes a number between hyphens is inserted before it to say that the double bond is between that atom...
, the suffix given to C=C double bonded alkenes) and "alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
" (which represents the enol's hydroxyl group).
Keto-enol tautomerism
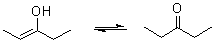
Deprotonation
Deprotonation is the removal of a proton from a molecule, forming the conjugate base.The relative ability of a molecule to give up a proton is measured by its pKa value. A low pKa value indicates that the compound is acidic and will easily give up its proton to a base...
on one side and protonated
Protonation
In chemistry, protonation is the addition of a proton to an atom, molecule, or ion. Some classic examples include*the protonation of water by sulfuric acid:*the protonation of isobutene in the formation of a carbocation:2C=CH2 + HBF4 → 3C+ + BF4−*the protonation of ammonia in the...
on another side, whereas a single bond and a double bond are exchanged. This is called keto-enol tautomerism
Keto-enol tautomerism
In organic chemistry, keto-enol tautomerism refers to a chemical equilibrium between a keto form and an enol . The enol and keto forms are said to be tautomers of each other...
.
The enol form is usually unstable, does not survive long, and changes into the keto (ketone). This is because oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
is more electronegative than carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
and thus forms stronger
Bond energy
In chemistry, bond energy is the measure of bond strength in a chemical bond. It is the heat required to break one Mole of molecules into their individual atoms. For example, the carbon-hydrogen bond energy in methane E is the enthalpy change involved with breaking up one molecule of methane into...
bonds
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
.
Tautomerism in multi-carbonyl compounds
In 1,3-dicarbonyl and 1,3,5-tricarbonyl compounds, however, the (mono-)enol form predominates. This is due to intramolecular hydrogen bondHydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
ing and possibly to an easy internal proton transfer.

Acetylacetone
Acetylacetone is an organic compound that famously exists in two tautomeric forms that rapidly interconvert. The less stable tautomer is a diketone formally named pentane-2,4-dione. The more common tautomer is the enol form. The pair of tautomers rapidly interconvert and are treated as a single...
, for example, 80 % enol form).
Enolates
When keto-enol tautomerism occurs the keto or enol is deprotonated and an anion, which is called the enolate, is formed as intermediateReaction intermediate
A reaction intermediate or an intermediate is a molecular entity that is formed from the reactants and reacts further to give the directly observed products of a chemical reaction. Most chemical reactions are stepwise, that is they take more than one elementary step to complete...
. Enolates can exist in quantitative amounts in strictly Brønsted acid free conditions, since they are generally very basic. In enolates the anionic charge is delocalized over the oxygen and the carbon
. Enolates are somewhat stabilized by this delocalization of the charge over three atoms. In older descriptions of bonding, particularly valence bond theory
Valence bond theory
In chemistry, valence bond theory is one of two basic theories, along with molecular orbital theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of the dissociated atoms combine to give individual chemical bonds...
this was explained by a phenomenon known as resonance
Resonance (chemistry)
In chemistry, resonance or mesomerism is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis formula...
.
| Keto-enol-tautomerism | ||
|---|---|---|
| |
||
| Interconversion between keto form and enolate; deprotonation of the α-C-atom. | Enolate anion, described is terms of resonance Resonance (chemistry) In chemistry, resonance or mesomerism is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis formula... . Left the carbanion Carbanion A carbanion is an anion in which carbon has an unshared pair of electrons and bears a negative charge usually with three substituents for a total of eight valence electrons. The carbanion exists in a trigonal pyramidal geometry. Formally a carbanion is the conjugate base of a carbon acid.where B... . |
Interconversion between enolate and enol; protonation of the enolate. |

Enolates in MO-theory

Molecular orbital theory
In chemistry, molecular orbital theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the whole molecule...
do away with the twin resonant structures, where electrons are assumed to be attached either to one or to two atoms only. Instead it is assumed that bonding electrons can very well be shared by three (or more) atoms. This is hard to picture when electrons are imagined as particles only. However, duality
Wave–particle duality
Wave–particle duality postulates that all particles exhibit both wave and particle properties. A central concept of quantum mechanics, this duality addresses the inability of classical concepts like "particle" and "wave" to fully describe the behavior of quantum-scale objects...
stipulates that they can also be seen as waves creating a standing wave pattern around all the atoms. Enolate presents an interesting and fairly simple example of the usefulness of this approach, because one can limit the discussion of the delocalization in terms of the number of contributing atomic orbitals to only three in this case: one pz orbital on each of the atoms C-C-O and leave the underlying skeleton of sigma bonds out of the picture.
When combining these three pz-orbitals, that were assumed involved in the "resonance" above, three molecular orbital
Molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first...
s can be formed. The one lowest in energy does not possess any vertical nodal planes separating the atoms. The energy of this orbital is lower than the energies of the atomic orbitals that it originates from. It therefore constitutes a bonding orbital as its formation stabilizes the electrons residing in it. Its bonding has π-symmetry because it inherits the horizontal nodal plane form the p-orbitals it is composed of. In the figure this is shown by the blue and red colors above and below the plane of the nuclei, indicating the opposite phase of the wave on either side of the nodal plane. The second lowest orbital is essentially non-bonding in nature; its energy is not very different from the energies of the parent atomic orbitals. It has one additional nodal plane that resides vertically (not quite) on the central atom. The result is the alternating four red and blue color regions in the figure. The last MO is higher in energy than the parent atomic orbitals and therefore antibonding in nature. It has two additional vertical nodes, leading to six red or blue regions.
Fortunately, the top antibonding orbital is empty in the ground state on the ion, because the number of electrons involved in the "resonance" is only four. This avoids having to pay the energetic price of destabilization that its elevated energy implies. The fact that there are only four electrons means that only the bonding and the non-bonding MO (π1 and π2) are filled. Thus there is one bonding pair spread over two bonding regions, adding half a bond order to the sigma bond already present between the atoms. In this model we would therefore expect two fairly equivalent bonds of bond order 1½ each, rather that one bond with order 2 and one bond with order one.
In this description only a single electronic structure is present, rather than two, in which the negative charge is distributed over the three atoms, albeit not entirely evenly. The π1 orbital puts much of the charge on the oxygen atom, the π2 one on the terminal carbon atom, leaving the middle atom with less of the negative charge.
Selective deprotonation in enolate forming
In ketones with α-hydrogens on both sides of the carbonyl carbon, selectivity of deprotonation may be achieved to generate two different enolate structures. At low temperatures (-78°C, i.e. dry ice bath), in aprotic solvents, and with bulky non-equilibrating bases (e.g. LDALithium diisopropylamide
Lithium diisopropylamide is the chemical compound with the formula [2CH]2NLi. Generally abbreviated LDA, it is a strong base used in organic chemistry for the deprotonation of weakly acidic compounds. The reagent has been widely accepted because it is soluble in non-polar organic solvents and it...
) the "kinetic" proton may be removed. The "kinetic" proton is the one which is sterically
Steric effects
Steric effects arise from the fact that each atom within a molecule occupies a certain amount of space. If atoms are brought too close together, there is an associated cost in energy due to overlapping electron clouds , and this may affect the molecule's preferred shape and reactivity.-Steric...
most accessible. Under thermodynamic conditions (higher temperatures, weak base, and protic solvent) equilibrium is established between the ketone and the two possible enolates, the enolate favoured is termed the "thermodynamic" enolate and is favoured because of its lower energy level than the other possible enolate. Thus, by choosing the optimal conditions to generate an enolate, one can increase the yield of the desired product while minimizing formation of undesired products.
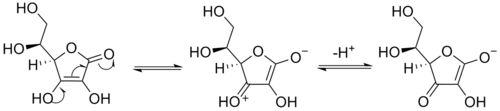
Enediols
Enediols are alkenes with a hydroxyl group on both sides of the C=C double bond. Enediols are reaction intermediateReaction intermediate
A reaction intermediate or an intermediate is a molecular entity that is formed from the reactants and reacts further to give the directly observed products of a chemical reaction. Most chemical reactions are stepwise, that is they take more than one elementary step to complete...
s in the Lobry-de Bruyn-van Ekenstein transformation
Lobry-de Bruyn-van Ekenstein transformation
In carbohydrate chemistry, the Lobry–de Bruyn–van Ekenstein transformation also known as the Lobry–de Bruyn–van-Alberda–van-Ekenstein transformation is the base or acid catalyzed transformation of an aldose into the ketose isomer or vice versa, with a tautomeric enediol as reaction intermediate....
.
Reductones
Enediols with a carbonyl group adjacent to the enediol group are called reductones. The enediol structure is stabilized by the resonanceResonance (chemistry)
In chemistry, resonance or mesomerism is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis formula...
, resulting from the tautomerism with the adjacent carbonyl. Therefore, the chemical equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
produces mainly the enediol form rather than the keto form. Reductones are strong reducing agents, thus efficacious antioxidant
Antioxidant
An antioxidant is a molecule capable of inhibiting the oxidation of other molecules. Oxidation is a chemical reaction that transfers electrons or hydrogen from a substance to an oxidizing agent. Oxidation reactions can produce free radicals. In turn, these radicals can start chain reactions. When...
s, and fairly strong acids.
Examples of reductones are tartronaldehyde, reductic acid and ascorbic acid.
| Examples of reductones | ||
|---|---|---|
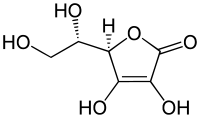 |
||
| Tartronaldehyde | Reductic acid | Ascorbic acid Ascorbic acid Ascorbic acid is a naturally occurring organic compound with antioxidant properties. It is a white solid, but impure samples can appear yellowish. It dissolves well in water to give mildly acidic solutions. Ascorbic acid is one form of vitamin C. The name is derived from a- and scorbutus , the... (Vitamin C) |

