Taxol total synthesis
Encyclopedia
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
is a major ongoing research effort in the total synthesis
Total synthesis
In organic chemistry, a total synthesis is, in principle, the complete chemical synthesis of complex organic molecules from simpler pieces, usually without the aid of biological processes. In practice, these simpler pieces are commercially available in bulk and semi-bulk quantities, and are often...
of paclitaxel
Paclitaxel
Paclitaxel is a mitotic inhibitor used in cancer chemotherapy. It was discovered in a U.S. National Cancer Institute program at the Research Triangle Institute in 1967 when Monroe E. Wall and Mansukh C. Wani isolated it from the bark of the Pacific yew tree, Taxus brevifolia and named it taxol...
(Taxol). This diterpenoid
Diterpene
Diterpenes are a type of terpenes composed of four isoprene units. They derive from geranylgeranyl pyrophosphate. Diterpenes form the basis for biologically important compounds such as retinol, retinal, and phytol...
is an important drug
Drug
A drug, broadly speaking, is any substance that, when absorbed into the body of a living organism, alters normal bodily function. There is no single, precise definition, as there are different meanings in drug control law, government regulations, medicine, and colloquial usage.In pharmacology, a...
in the treatment of cancer
Cancer
Cancer , known medically as a malignant neoplasm, is a large group of different diseases, all involving unregulated cell growth. In cancer, cells divide and grow uncontrollably, forming malignant tumors, and invade nearby parts of the body. The cancer may also spread to more distant parts of the...
but also expensive because the compound is harvested from a scarce resource, namely the Pacific yew
Taxus brevifolia
Taxus brevifolia is a conifer native to the Pacific Northwest of North America. It ranges from southernmost Alaska south to central California, mostly in the Pacific Coast Ranges, but with an isolated disjunct population in southeast British Columbia, most notably occurring on Zuckerberg Island...
(Taxus brevifolia). Not only is the synthetic reproduction of the compound itself of great commercial and scientific importance, but it also opens the way to paclitaxel derivatives not found in nature but with greater potential.
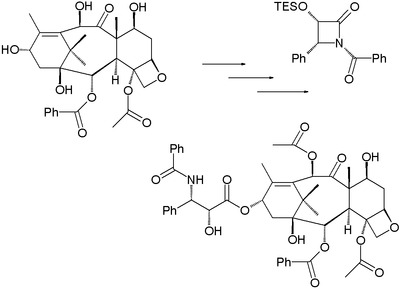
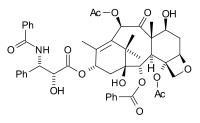
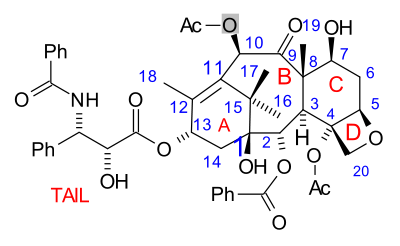
The paclitaxel molecule consists of a tetracyclic core called baccatin III and an amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
tail. The core rings are conveniently called (from left to right) ring A (a cyclohexene
Cyclohexene
Cyclohexene is a hydrocarbon with the formula C6H10. This cycloalkene is a colorless liquid with a sharp smell. It is an intermediate in various industrial processes...
), ring B (a cyclooctane
Cyclooctane
Cyclooctane is a cycloalkane with the molecular formula 8. It is a simple colourless hydrocarbon, but it is often a reference compound for saturated eight-membered ring compounds in general.- Conformation :...
), ring C (a cyclohexane
Cyclohexane
Cyclohexane is a cycloalkane with the molecular formula C6H12. Cyclohexane is used as a nonpolar solvent for the chemical industry, and also as a raw material for the industrial production of adipic acid and caprolactam, both of which being intermediates used in the production of nylon...
) and ring D (an oxetane
Oxetane
Oxetane, or 1,3-propylene oxide, is an heterocyclic organic compound with the molecular formula C3H6O, having a four-membered ring with three carbon atoms and one oxygen atom....
).
The paclitaxel drug development process took over 40 years. The anti-tumor activity of a bark extract of the Pacific yew tree was discovered in 1963 as a follow up of a US government plant screening program already in existence 20 years before that. The active substance responsible for the anti-tumor activity was discovered in 1969 and structure elucidation was completed in 1971. Robert A Holton of Florida State University
Florida State University
The Florida State University is a space-grant and sea-grant public university located in Tallahassee, Florida, United States. It is a comprehensive doctoral research university with medical programs and significant research activity as determined by the Carnegie Foundation...
succeeded in the total synthesis of paclitaxel in 1994, a project that he had started in 1982. In 1989 Holton had also developed a semisynthetic route to paclitaxel starting from 10-deacetylbaccatin III. This compound is a biosynthetic precursor and is found in larger quantities than paclitaxel itself in Taxus baccata
Taxus baccata
Taxus baccata is a conifer native to western, central and southern Europe, northwest Africa, northern Iran and southwest Asia. It is the tree originally known as yew, though with other related trees becoming known, it may be now known as the English yew, or European yew.-Description:It is a small-...
(the European Yew). In 1990
Bristol-Myers Squibb
Bristol-Myers Squibb
Bristol-Myers Squibb , often referred to as BMS, is a pharmaceutical company, headquartered in New York City. The company was formed in 1989, following the merger of its predecessors Bristol-Myers and the Squibb Corporation...
bought a licence to the patent for this process which in the years to follow earned Florida State University and Holton (with a 40% take) over 200 million US dollars.
Total synthesis
Some of the efforts are truly synthetic but in others a precursor molecule found in nature is included. The key data are collected below. What all strategies have in common is synthesis of the baccatin molecule followed by last stage addition of the tail, a process (except for one) based on the Ojima lactam
Ojima lactam
The Ojima lactam is an organic compound of some importance in the commercial production of Taxol. This lactam was first synthesized by Iwao Ojima . The organic synthesis is an illustration of asymmetric synthesis via a chiral auxiliary....
.
- Holton Taxol total synthesisHolton Taxol total synthesisThe Holton Taxol total synthesis, published by Robert A. Holton and his group at Florida State University in 1994 was the first total synthesis of Taxol ....
- year: 1994 - precursor: PatchoulolPatchoulolPatchoulol or patchouli alcohol is a terpene extracted from Patchouli. The -optical isomer is one of the organic compounds responsible for the typical patchouli scent. Patchoulol is also used in the synthesis of the chemotherapy drug Taxol....
- strategy: linear synthesis AB then C then D - references: see related article - Nicolaou Taxol total synthesisNicolaou Taxol total synthesisThe Nicolaou Taxol total synthesis, published by K. C. Nicolaou and his group in 1994 concerns the total synthesis of Taxol. This organic synthesis was included in Nicolaou's book, 'Classics in Total Synthesis'....
- year: 1994 - precursor: Mucic acidMucic acidMucic acid, C6H10O8 or HOOC-4-COOH, is obtained by nitric acid oxidation of galactose or galactose-containing compounds like lactose, dulcite, quercite, and most varieties of gum....
strategy: convergent synthesisConvergent synthesisIn chemistry a convergent synthesis is a strategy that aims to improve the efficiency of multi-step chemical synthesis, most often in organic synthesis...
A and C merge to ABC then D - references: see related article - Danishefsky Taxol total synthesisDanishefsky Taxol total synthesisThe Danishefsky Taxol total synthesis in organic chemistry is an important third Taxol synthesis published by the group of Samuel Danishefsky in 1996...
- year: 1996 - precursor: Wieland-Miescher ketoneWieland-Miescher ketoneThe Wieland–Miescher ketone is a racemic bicyclic diketone and is a versatile synthon which has so far been employed in the total synthesis of more than 50 natural products, predominantly sesquiterpenoids, diterpenes and steroids possessing possible biological properties including anticancer,...
strategy: convergent synthesisConvergent synthesisIn chemistry a convergent synthesis is a strategy that aims to improve the efficiency of multi-step chemical synthesis, most often in organic synthesis...
C merges with D then with A merges to ABCD - references: See related article - Wender Taxol total synthesisWender Taxol total synthesisThe Wender Taxol total synthesis in organic chemistry describes a Taxol total synthesis by the group of Paul A. Wender at Stanford University published in 1997. This synthesis has much in common with the Holton Taxol total synthesis in that it is a linear synthesis starting from a naturally...
- year: 1997 - precursor: PinenePinenePinene is a bicyclic monoterpene chemical compound. There are two structural isomers of pinene found in nature: α-pinene and β-pinene. As the name suggests, both forms are important constituents of pine resin; they are also found in the resins of many other conifers, as well as in non-coniferous...
strategy: linear synthesis AB then C then D - references: - Kuwajima Taxol total synthesisKuwajima Taxol total synthesisThe Kuwajima Taxol total synthesis by the group of Isao Kuwajima of the Tokyo Institute of Technology is one of several efforts in taxol total synthesis published in the 1990s...
I. Kuwajima, - year: 1998 - precursor: synthetic building blocks strategy: linear synthesis A then B then C then D - Mukaiyama Taxol total synthesisMukaiyama Taxol total synthesisThe Mukaiyama taxol total synthesis published by the group of Teruaki Mukaiyama of the Tokyo University of Science between 1997 and 1999 was the 6th successful taxol total synthesis. The total synthesis of Taxol is considered a hallmark in organic synthesis....
T. Mukaiyama, - year: 1998 - Precursor: L-serine strategy: linear synthesis B, then C, then A then D. References: see related article. - Takahashi Taxol racemic fomal total synthesis T. Takahashi, - year: 2006 - Precursor: geraniolGeraniolGeraniol is a monoterpenoid and an alcohol. It is the primary part of rose oil, palmarosa oil, and citronella oil . It also occurs in small quantities in geranium, lemon, and many other essential oils. It appears as a clear to pale-yellow oil that is insoluble in water, but soluble in most common...
strategy: convergent synthesisConvergent synthesisIn chemistry a convergent synthesis is a strategy that aims to improve the efficiency of multi-step chemical synthesis, most often in organic synthesis...
A and C merge to ABC then D
Semisynthesis
The commercial semisynthesis (by Bristol-Myers SquibbBristol-Myers Squibb
Bristol-Myers Squibb , often referred to as BMS, is a pharmaceutical company, headquartered in New York City. The company was formed in 1989, following the merger of its predecessors Bristol-Myers and the Squibb Corporation...
) of paclitaxel starting from 10-deacetylbaccatin III (isolated from the European yew) is based on tail addition of the so-called Ojima lactam
Ojima lactam
The Ojima lactam is an organic compound of some importance in the commercial production of Taxol. This lactam was first synthesized by Iwao Ojima . The organic synthesis is an illustration of asymmetric synthesis via a chiral auxiliary....
to its free hydroxyl group:
Another commercial semisynthesis (by the company Natural Pharmaceuticals) relies on the isolation of a group of paclitaxel derivatives isolated from primary ornamental taxanes. These derivatives have the same skeleton as paclitaxel except for the organic residue R of the terminal tail amide group which can be phenyl, or propyl
Propyl
In organic chemistry, propyl is a three-carbon alkyl substituent with chemical formula -C3H7. It is the substituent form of the alkane propane...
or pentyl
Pentyl
In organic chemistry, pentyl is a five-carbon alkyl substituent with chemical formula -C5H11. It is the substituent form of the alkane pentane. In older literature, the common non-systematic name "amyl" was often used for the pentyl group....
(among others) whereas in paclitaxel it is an explicit phenyl group. The semisynthesis consists of conversion of the amide group to an amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
with Schwartz's reagent
Schwartz's Reagent
Schwartz's reagent is the common name for the chemical compound with the formula 2ZrHCl, sometimes described zirconocene hydrochloride or zirconocene chloride hydride and is named after Jeffrey Schwartz, who is currently a professor in Chemistry at Princeton University...
through an imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
followed by acidic workup and a benzoylation.
In the production process Michigan
Michigan
Michigan is a U.S. state located in the Great Lakes Region of the United States of America. The name Michigan is the French form of the Ojibwa word mishigamaa, meaning "large water" or "large lake"....
grown yews which mature in 8 years are periodically topped and dried. This material is shipped to Mexico
Mexico
The United Mexican States , commonly known as Mexico , is a federal constitutional republic in North America. It is bordered on the north by the United States; on the south and west by the Pacific Ocean; on the southeast by Guatemala, Belize, and the Caribbean Sea; and on the east by the Gulf of...
for a first extraction step (10% paclitaxel content) and then to Canada
Canada
Canada is a North American country consisting of ten provinces and three territories. Located in the northern part of the continent, it extends from the Atlantic Ocean in the east to the Pacific Ocean in the west, and northward into the Arctic Ocean...
for further purification to 95% purity. The semisynthesis to final product takes place in China
China
Chinese civilization may refer to:* China for more general discussion of the country.* Chinese culture* Greater China, the transnational community of ethnic Chinese.* History of China* Sinosphere, the area historically affected by Chinese culture...
.
Biosynthesis
The biosynthetic pathway to paclitaxel has been investigated and consists of approximately 20 enzymatic steps. The complete scheme is still unavailable. The segments that are known are very different from the synthetic pathways tried thus far (Scheme 1). The starting compound is geranylgeranyl diphosphate
Pyrophosphate
In chemistry, the anion, the salts, and the esters of pyrophosphoric acid are called pyrophosphates. Any salt or ester containing two phosphate groups is called a diphosphate. As a food additive, diphosphates are known as E450.- Chemistry :...
2 which is a dimer of geraniol
Geraniol
Geraniol is a monoterpenoid and an alcohol. It is the primary part of rose oil, palmarosa oil, and citronella oil . It also occurs in small quantities in geranium, lemon, and many other essential oils. It appears as a clear to pale-yellow oil that is insoluble in water, but soluble in most common...
1. This compound already contains all the required 20 carbon atoms for the paclitaxel skeleton. More ring closing through intermediate 3 (taxadiene) leads to taxusin 4. The two main reasons why this type of synthesis is not feasible in the laboratory is that nature does a much better job controlling stereochemistry
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
and a much better job activating a hydrocarbon skeleton with oxygen substituents for which Cytochrome P450 is responsible in some of the oxygenations. Intermediate 5 is called 10-deacetylbaccatin III.
A biochemical kilogram-scale production of taxadiene has been reported using genetically engineered E. coli
Escherichia coli
Escherichia coli is a Gram-negative, rod-shaped bacterium that is commonly found in the lower intestine of warm-blooded organisms . Most E. coli strains are harmless, but some serotypes can cause serious food poisoning in humans, and are occasionally responsible for product recalls...
.
External links
- Paclitaxel Total Syntheses @ SynArchive.com
- Holton founded http://www.taxolog.com for Taxol research
- The complete Taxol story from Chemical & Engineering NewsChemical & Engineering NewsChemical & Engineering News is a weekly magazine published by the American Chemical Society, providing professional and technical information in the fields of chemistry and chemical engineering...
: Article - Caltech taxol overview
- extensive Florida State University article
- story of taxol total synthesis