Acetal
Encyclopedia
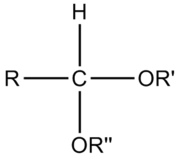
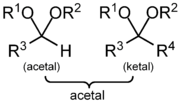
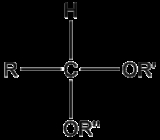
An acetal is a molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
with two single-bonded oxygen atoms attached to the same carbon atom.
Traditional usages distinguish ketals from acetals (whereas a ketal has two carbon-bonded R groups and is formally derived from a ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
, an acetal has one or both carbon-bonded R groups as a hydrogen and is formally derived from an aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
). Current IUPAC terminology classifies ketals as a subset of acetals.
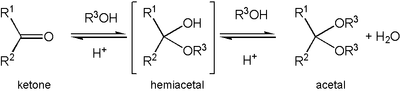
Formation of an acetal occurs when the hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
group of a hemiacetal
Hemiacetal
Hemiacetals and hemiketals are compounds that are derived from aldehydes and ketones respectively. The Greek word hèmi means half...
becomes protonated and is lost as water. The carbocation
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
ion that is produced is then rapidly attacked by a molecule of alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
. Loss of the proton from the attached alcohol gives the acetal.
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
as with esters. As a reaction to create an acetal proceeds, water must be removed from the reaction mixture, for example, with a Dean-Stark apparatus
Dean-Stark apparatus
For the video game character, see Wild Arms 5thumb|right|A Dean-Stark apparatus in use; aluminum foil is used to reduce radiative heat lossesThe Dean-Stark apparatus or Dean-Stark receiver or distilling trap is a piece of laboratory glassware used in synthetic chemistry to collect water from a...
, lest it will hydrolyse the product back to the hemiacetal. The formation of acetals reduces the total number of molecules present and therefore is not favourable with regards to entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
. A way to improve this is to use an orthoester
Orthoester
In organic chemistry, an orthoester is a functional group containing three alkoxy groups attached to one carbon atom, i.e. with the general formula RC3. The name can also refer to any organic compound that contains this functional group. An example of an orthoester is ethyl orthoacetate, CH3C3,...
as a source of alcohol. Aldehydes and ketones undergo a process called acetal exchange with orthoesters to give acetals. Water produced along with the acetal product is used up in hydrolysing the orthoester
Orthoester
In organic chemistry, an orthoester is a functional group containing three alkoxy groups attached to one carbon atom, i.e. with the general formula RC3. The name can also refer to any organic compound that contains this functional group. An example of an orthoester is ethyl orthoacetate, CH3C3,...
and producing more alcohol to be used in the reaction.
Most glycosidic bond
Glycosidic bond
In chemistry, a glycosidic bond is a type of covalent bond that joins a carbohydrate molecule to another group, which may or may not be another carbohydrate....
s in carbohydrate
Carbohydrate
A carbohydrate is an organic compound with the empirical formula ; that is, consists only of carbon, hydrogen, and oxygen, with a hydrogen:oxygen atom ratio of 2:1 . However, there are exceptions to this. One common example would be deoxyribose, a component of DNA, which has the empirical...
s and other polysaccharide
Polysaccharide
Polysaccharides are long carbohydrate molecules, of repeated monomer units joined together by glycosidic bonds. They range in structure from linear to highly branched. Polysaccharides are often quite heterogeneous, containing slight modifications of the repeating unit. Depending on the structure,...
s are acetal linkages. Acetaldehyde diethyl acetal is an important flavouring compound in distilled beverage
Distilled beverage
A distilled beverage, liquor, or spirit is an alcoholic beverage containing ethanol that is produced by distilling ethanol produced by means of fermenting grain, fruit, or vegetables...
s.
The plastic known as acetal is a polyacetal of formaldehyde
Formaldehyde
Formaldehyde is an organic compound with the formula CH2O. It is the simplest aldehyde, hence its systematic name methanal.Formaldehyde is a colorless gas with a characteristic pungent odor. It is an important precursor to many other chemical compounds, especially for polymers...
.
Acetals are used as protecting group
Protecting group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group in order to obtain chemoselectivity in a subsequent chemical reaction...
s for carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
groups in organic synthesis as they are stable with respect to hydrolysis by bases and with respect to many oxidizing and reducing agents.
Examples
- DimethoxymethaneDimethoxymethaneDimethoxymethane, also called methylal, is a clear colorless flammable liquid with a low boiling point, low viscosity and an excellent dissolving power. It has a chloroform-like odor and a pungent taste. It is the dimethyl acetal of formaldehyde...
, a solvent - DioxolaneDioxolaneDioxolane is a heterocyclic acetal with the chemical formula 2O2CH2. It is related to tetrahydrofuran by interchange of one oxygen for a CH2 group. The corresponding saturated 6-membered C4O2 rings are called dioxanes. The isomeric 1,2-dioxolane is an peroxide...
- MethylenedioxyMethylenedioxyMethylenedioxy in the field of chemistry, particularly in organic chemistry, is the name for a functional group with the structural formula R-O-CH2-O-R' which is connected to the rest of a molecule by two chemical bonds. The methylenedioxy group consists of two oxygen atoms connected to a methylene...
group - MetaldehydeMetaldehydeMetaldehyde is an organic compound with the formula 4. It is commonly used as a pesticide against slugs, snails, and other gastropods. It is the cyclic tetramer of acetaldehyde.-Production and properties:...
- ParaldehydeParaldehydeParaldehyde is the cyclic trimer of acetaldehyde molecules. Formally, it is a derivative of 1,3,5-trioxane. The corresponding tetramer is metaldehyde. A colourless liquid, it is sparingly soluble in water and highly soluble in alcohol. Paraldehyde slowly oxidizes in air, turning brown and producing...
- 1,3,5-Trioxane1,3,5-Trioxane1,3,5-Trioxane, sometimes also called trioxin, is a chemical compound with molecular formula C3H6O3. It is a stable cyclic trimer of formaldehyde, and one of the two trioxane isomers; its molecular backbone consists of a six membered ring with three carbon atoms alternating with three oxygen...

