
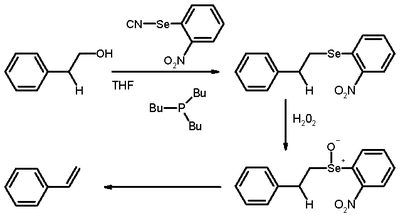
Grieco elimination
Encyclopedia
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
describing the elimination reaction
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
of an aliphatic primary alcohol
Primary alcohol
A primary alcohol is an alcohol which has the hydroxyl radical connected to a primary carbon. It can also be defined as a molecule containing a “–CH2OH” group.Examples include ethanol and butanol....
through a selenide
Selenide
A selenide is a chemical compound in which selenium serves as an anion with oxidation number of −2 , much as sulfur does in a sulfide. The chemistry of the selenides and sulfides are similar....
to a terminal alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
.
The alcohol first reacts with o-nitrophenylselenocyanate and tributylphosphine
Tributylphosphine
Tributylphosphine, formula P or PBu, is a tertiary phosphine, most commonly encountered as a ligand in transition metal complexes. It is an oily liquid at room temperature, with a nauseating odor. It reacts slowly with atmospheric oxygen, and rapidly with other oxidizing agents, to give the...
to a selenide
Selenide
A selenide is a chemical compound in which selenium serves as an anion with oxidation number of −2 , much as sulfur does in a sulfide. The chemistry of the selenides and sulfides are similar....
in a nucleophilic substitution
Nucleophilic substitution
In organic and inorganic chemistry, nucleophilic substitution is a fundamental class of reactions in which an electron nucleophile selectively bonds with or attacks the positive or partially positive charge of an atom or a group of atoms called the leaving group; the positive or partially positive...
on electron deficient selenium
Selenium
Selenium is a chemical element with atomic number 34, chemical symbol Se, and an atomic mass of 78.96. It is a nonmetal, whose properties are intermediate between those of adjacent chalcogen elements sulfur and tellurium...
. In the second step the selenide is oxidized with hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
to a selenoxide in which elimination takes place with expulsion of a selenol
Selenol
Selenols are organic compounds that contain the functional group with the connectivity C-Se-H. Selenols are sometimes also called selenamercaptans, selenathiols, and selenothiols. Selenols are one of the principal classes of organoselenium compounds...
in a fashion similar to that of the Cope elimination. This reaction takes part in the synthesis of ring C of the Danishefsky Taxol synthesis.

