Silyl enol ether
Encyclopedia

Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
are a class of organic compound
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
s that share a common functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
composed of an enolate bonded through its oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
terminus to an organosilicon
Organosilicon
Organosilicon compounds are organic compounds containing carbon silicon bonds. Organosilicon chemistry is the corresponding science exploring their properties and reactivity.Like carbon, the organically bound silicon is tetravalent and tetrahedral...
group.
Silyl enol ethers are important intermediates in organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
.
Organic synthesis
- Trimethylsilyl enol ethers can be prepared from ketones in presence of a strong base and trimethylsilyl chlorideTrimethylsilyl chlorideTrimethylsilyl chloride, also known as chlorotrimethylsilane is a silyl halide, with a variety of different uses in chemistry. It has the formula 3SiCl, and under standard conditions it is a colourless liquid, which is stable in the absence of water...
or a weak base and trimethylsilyl triflate. - Silyl enol ether can form by capturing any enolate formed in a nucleophilic conjugate additionNucleophilic conjugate additionNucleophilic conjugate addition is a type of organic reaction. Ordinary nucleophilic additions or 1,2-nucleophilic additions deal mostly with additions to carbonyl compounds. Simple alkene compounds do not show 1,2 reactivity due to lack of polarity, unless the alkene is activated with special...
. - A rather exotic way to generate silyl enol ethers is via the Brook rearrangementBrook rearrangementThe Brook rearrangement in organic chemistry is a rearrangement reaction in which a organosilyl group switches position with a hydroxyl proton over a carbon to oxygen covalent bond under the influence of a base . It is named for the Canadian chemist Adrian Gibbs Brook...
of appropriate substrates.
Organic reactions
Silyl enol ethers react as nucleophileNucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
s in:
- Mukaiyama aldol additionMukaiyama aldol additionThe Mukaiyama aldol addition is an organic reaction and a type of aldol reaction between a silyl enol ether and an aldehyde catalyzed by a Lewis acid. This choice of reactants allows for a crossed aldol reaction between an aldehyde and a ketone or a different aldehyde without self-condensation of...
- Michael reactionMichael reactionThe Michael reaction or Michael addition is the nucleophilic addition of a carbanion or another nucleophile to an alpha, beta unsaturated carbonyl compound. It belongs to the larger class of conjugate additions. This is one of the most useful methods for the mild formation of C-C bonds...
s - AlkylationAlkylationAlkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion or a carbene . Alkylating agents are widely used in chemistry because the alkyl group is probably the most common group encountered in...
s - HaloketoneHaloketoneA haloketone in organic chemistry is a functional group consisting of a ketone group or more general a carbonyl group with a α-halogen substituent. The general structure is RR'CCR where R is an alkyl or aryl residue and X any one of the halogens...
formation with halogenHalogenThe halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
s - AcyloinAcyloinAcyloins are a class of organic compounds in organic chemistry sharing a common functional group consisting of a hydroxyl group placed on the α-position of a carbonyl group.- Nomenclature :Common types of ketols include:...
formation by organic oxidation with an electrophilic source of oxygen such as an oxaziridineOxaziridineAn oxaziridine is an organic molecule that features a three-membered heterocycle containing oxygen, nitrogen, and carbon.-History:Oxaziridine derivatives were first synthesized in the mid 1950s by Emmons and subsequently by Krimm and Horner and Jürgens...
or mCPBA
Saegusa–Ito oxidation
In the Saegusa–Ito oxidation certain silyl enol ethers are oxidized to enoneEnone
An enone is an unsaturated chemical compound or functional group consisting of a conjugated system of an alkene and a ketone. The simplest enone is methyl vinyl ketone or CH2=CHCOCH3....
s with palladium(II) acetate
Palladium(II) acetate
Palladium acetate is a chemical compound of palladium described by the formula Pd2 or Pd2. It is considered more reactive than the analogous platinum compound...
. In the original publication equal amounts of palladium and 1,4-benzoquinone
1,4-Benzoquinone
1,4-Benzoquinone, commonly known as para-quinone, is a chemical compound with the formula C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine, bleach, and hot plastic. Impure samples are often dark-colored due to the presence...
are used to achieve the reaction with the benzoquinone acting as a co-oxidant. The intermediate is an oxo-allylpalladium complex.
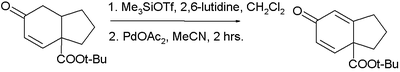
In one application a dienenone is synthesized in two steps from a cyclohexanone:
Ketene silyl acetals
Ketene silyl acetals are related compounds formally derived from keteneKetene
A ketene is an organic compound of the form R'RC=C=O. The term is also used specifically to mean ethenone, the simplest ketene, where R' and R are hydrogen atoms.Ketenes were first studied as a class by Hermann Staudinger.-Formation:...
s and acetal
Acetal
An acetal is a molecule with two single-bonded oxygen atoms attached to the same carbon atom.Traditional usages distinguish ketals from acetals...
s with general structure R-C=C(OSiR3)(OR').