Hydrogenation
Encyclopedia
Hydrogenation, to treat with hydrogen, also a form of chemical reduction
, is a chemical reaction
between molecular hydrogen
(H2) and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce
or saturate
organic compound
s. Hydrogenation typically constitutes the addition of pairs of hydrogen
atom
s to a molecule, generally an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogen
adds to double and triple bond
s in hydrocarbon
s.
Because of the importance of hydrogen, many related reactions have been developed for its use. Most hydrogenations use gaseous hydrogen (H2), but some involve the alternative sources of hydrogen, not H2: these processes are called transfer hydrogenation
s. The reverse reaction, removal of hydrogen from a molecule, is called dehydrogenation
. A reaction where bonds are broken while hydrogen is added is called hydrogenolysis
, a reaction that may occur to carbon-carbon and carbon-heteroatom (oxygen
, nitrogen
or halogen
) bonds. Hydrogenation differs from protonation
or hydride
addition: in hydrogenation, the products have the same charge as the reactants.
An illustrative example of a hydrogenation reaction is the addition of hydrogen to maleic acid
to form succinic acid
. Numerous important applications of this petrochemical
are found in pharmaceutical and food industries. Hydrogenation of unsaturated fat
s produces saturated fat
s and, in some cases, trans fat
s.
substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst.
in the protypical reaction:
Hydrogenation is sensitive to steric hindrance explaining the selectivity for reaction with the exocyclic double bond but not the internal double bond.
An important characteristic of alkene and alkyne hydrogenations, both the homogeneously and heterogeneously catalyzed versions, is that hydrogen addition occurs with "syn addition
", with hydrogen entering from the least hindered side. Typical substrates are listed in the table
, palladium
, rhodium
, and ruthenium
form highly active catalysts, which operate at lower temperatures and lower pressures of H2. Non-precious metal catalysts, especially those based on nickel (such as Raney nickel
and Urushibara nickel) have also been developed as economical alternatives, but they are often slower or require higher temperatures. The trade-off is activity (speed of reaction) vs. cost of the catalyst and cost of the apparatus required for use of high pressures. Notice that the Raney-nickel catalysed hydrogenations require high pressures:
Two broad families of catalysts are known - homogeneous catalysts and heterogeneous catalysts. Homogeneous catalysts dissolve in the solvent that contains the unsaturated substrate. Heterogeneous catalysts are solids that are suspended in the same solvent with the substrate or are treated with gaseous substrate.
-based compound known as Wilkinson's catalyst
and the iridium
-based Crabtree's catalyst
. An example is the hydrogenation of carvone
:
Hydrogenation is sensitive to steric hindrance explaining the selectivity for reaction with the exocyclic double bond but not the internal double bond.
The activity and selectivity of homogeneous catalysts is adjusted by changing the ligands. For prochiral
substrates, the selectivity of the catalyst can be adjusted such that one enantiomeric product is favored. Asymmetric hydrogenation is also possible via heterogeneous catalysis on a metal that is modified by a chiral ligand.
. Different faces
of a crystalline heterogeneous catalyst display distinct activities, for example. Similarly, heterogeneous catalysts are affected by their supports, i.e. the material upon with the heterogeneous catalyst is bound.
In many cases, highly empirical modifications involve selective "poisons". Thus, a carefully chosen catalyst can be used to hydrogenate some functional groups without affecting others, such as the hydrogenation of alkenes without touching aromatic rings, or the selective hydrogenation of alkynes to alkenes using Lindlar's catalyst
. For example, when the catalyst palladium
is placed on barium sulfate
and then treated with quinoline
, the resulting catalyst reduces alkynes only as far as alkenes. The Lindlar catalyst has been applied to the conversion of phenylacetylene
to styrene.
Asymmetric hydrogenation is also possible via heterogeneous catalysis on a metal that is modified by a chiral ligand.
.
s include hydrazine
, dihydronaphthalene, dihydroanthracene, isopropanol, and formic acid
. In organic synthesis
, transfer hydrogenation
is useful for the asymmetric reduction of polar unsaturated substrates, such as ketone
s, aldehyde
s, and imine
s.
reaction. In the hydrogenation of vegetable oils and fatty acids, for example, the heat released is about 25 kcal per mole (105 kJ/mol), sufficient to raise the temperature of the oil by 1.6-1.7 °C per iodine number
drop. The mechanism
of metal-catalyzed hydrogenation of alkenes and alkynes has been extensively studied. First of all isotope labeling using deuterium
confirms the regiochemistry of the addition:
In the second step, the metallointermediate formed is a saturated compound that can rotate and then break down, again detaching the alkene from the catalyst. Consequently, contact with a hydrogenation catalyst necessarily causes cis-trans-isomerization. This is a problem in partial hydrogenation, while in complete hydrogenation the produced trans-alkene is eventually hydrogenated.
For aromatic substrates, the first bond is hardest to hydrogenate because of the free energy penalty for breaking the aromatic system. The product of this is a cyclohexadiene, which is extremely active and cannot be isolated; in conditions reducing enough to break the aromatization, it is immediately reduced to a cyclohexene. The cyclohexene
is ordinarily reduced immediately to a fully saturated cyclohexane, but special modifications to the catalysts (such as the use of the anti-solvent water on ruthenium) can preserve some of the cyclohexene, if that is a desired product.
Preceding the oxidative addition of H2 is the formation of a dihydrogen complex
.
Oxygen can be partially hydrogenated to give hydrogen peroxide
, although this process has not been commercialized.
In petrochemical processes, hydrogenation is used to convert alkenes and aromatics into saturated alkanes (paraffins) and cycloalkanes (naphthenes), which are less toxic and less reactive. For example, mineral turpentine
is usually hydrogenated. Hydrocracking of heavy residues into diesel is another application. In isomerization and catalytic reforming
processes, some hydrogen pressure is maintained to hydrogenolyze
coke
formed on the catalyst and prevent its accumulation.
Xylitol
, a polyol
, is produced by hydrogenation of the sugar xylose
, an aldehyde.
s. Complete hydrogenation converts unsaturated fatty acid
s to saturated
ones. In practice the process is not usually carried to completion. Since the original oils usually contain more than one carbon-carbon double bond
per molecule (that is, they are polyunsaturated), the result is usually described as partially hydrogenated vegetable oil; that is some, but usually not all, of the carbon-carbon double bonds in each molecule have been reduced. This is done by restricting the amount of hydrogen (or reducing agent) allowed to react with the fat.
Hydrogenation results in the conversion of liquid vegetable oils to solid or semi-solid fats, such as those present in margarine
. Changing the degree of saturation of the fat changes some important physical properties such as the melting range, which is why liquid oils become semi-solid. Solid or semi-solid fats are preferred for baking because the way the fat mixes with flour produces a more desirable texture in the baked product. Because partially hydrogenated vegetable oils are cheaper than animal source fats, they are available in a wide range of consistencies, and have other desirable characteristics (e.g., increased oxidative stability/longer shelf life), they are the predominant fats used as shortening
in most commercial baked goods.
s predominates in the unprocessed fats in most edible fat sources, but incomplete hydrogenation partially converts these molecules to trans isomers, which have been implicated in circulatory diseases including heart disease
(see trans fat
s). The conversion from cis to trans bonds is favored because the trans configuration has lower energy than the natural cis one. At equilibrium, the trans/cis isomer ratio is about 2:1. Food legislation in the US and codes of practice in EU have long required labels declaring the fat content of foods in retail trade and, more recently, have also required declaration of the trans fat content. Trans fats are banned in Denmark
and New York City
.
catalyzed
addition of hydrogen to oxygen in the Döbereiner's lamp
, a device commercialized as early as 1823. The French chemist Paul Sabatier
is considered the father of the hydrogenation process. In 1897, building on the earlier work of James Boyce
, an American chemist working in the manufacture of soap products, he discovered that the introduction of a trace of nickel as a catalyst facilitated the addition of hydrogen to molecules of gaseous hydrocarbons in what is now known as the Sabatier process. For this work Sabatier shared the 1912 Nobel Prize in Chemistry
. Wilhelm Normann
was awarded a patent in Germany in 1902 and in Britain in 1903 for the hydrogenation of liquid oils, which was the beginning of what is now a world wide industry. The commercially important Haber-Bosch process, first described in 1905, involves hydrogenation of nitrogen. In the Fischer-Tropsch process
, reported in 1922 carbon monoxide, which is easily derived from coal, is hydrogenated to liquid fuels.
Also in 1922, Voorhees and Adams described an apparatus for performing hydrogenation under pressures above one atmosphere. The Parr shaker, the first product to allow hydrogenation using elevated pressures and temperatures, was commercialized in 1926 based on Voorhees and Adams’ research and remains in widespread use. In 1924 Murray Raney
developed a nickel fine powder catalyst named after him which is still widely used in hydrogenation reactions such as conversion of nitriles to amines or the production of margarine. In 1938, Otto Roelen
described the oxo process which involves the addition of both hydrogen and carbon monoxide to alkenes, giving aldehydes. Since this process entails C-C coupling, it and its many variations (see carbonylation
) remains highly topical into the new decade. The 1960s witnessed the development of homogeneous catalysts, e.g., Wilkinson's catalyst
. In the 1980s, the Noyori asymmetric hydrogenation
represented one of the first applications of hydrogenation in asymmetric synthesis, a growing application in the production of fine chemicals.
. Some metal-free catalytic systems have been investigated in academic research. One such system for reduction of ketone
s consists of tert-butanol
and potassium tert-butoxide
and very high temperatures. The reaction depicted below describes the hydrogenation of benzophenone
:
A chemical kinetics
study found this reaction is first-order
in all three reactants suggesting a cyclic 6-membered transition state
.
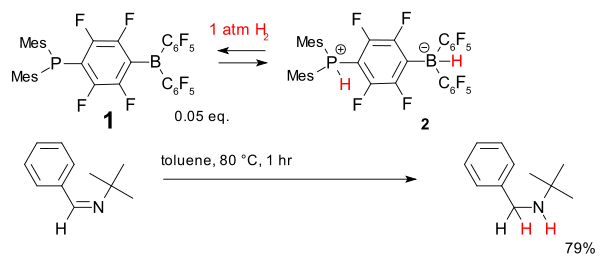
Another system for metal-free hydrogenation is based on the phosphine
-borane
, compound 1, which has been called a frustrated Lewis pair
. It reversibly accepts dihydrogen at relatively low temperatures to form the phosphonium
borate
2 which can reduce simple hindered imine
s.
The reduction of nitrobenzene
to aniline
has been reported to be catalysed by fullerene
, its mono-anion, atmospheric hydrogen and UV light.
or argon
gas and sealing the mixture with a penetrable rubber seal. Hydrogen gas is then supplied from a H2-filled balloon
. The resulting three phase mixture is agitated to promote mixing. Hydrogen uptake can be monitored, which can be useful for monitoring progress of a hydrogenation. This is achieved by either using a graduated tube containing a coloured liquid, usually aqueous copper sulfate or with gauges
for each reaction vessel.
of protecting groups and the reduction of aromatic systems proceed extremely sluggishly at atmospheric temperature and pressure, pressurised systems are popular. In these cases, catalyst is added to a solution of reactant under an inert atmosphere in a pressure vessel
. Hydrogen is added directly from a cylinder or built in laboratory hydrogen source, and the pressurized slurry is mechanically rocked to provide agitation or a spinning basket is used. Heat may also be used, as the pressure compensates for the associated reduction in gas solubility.
technology, this technique allows the application of pressures from atmospheric to 1,450 PSI. Elevated temperatures may also be used. At the bench scale, systems use a range of pre-packed catalysts which eliminates the need for weighing and filtering pyrophoric catalysts.
Gas Liquid Induction Reactors (Hydrogenator) are also used for carrying out catalytic hydrogenation.
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
, is a chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
between molecular hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
(H2) and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
or saturate
Saturation (chemistry)
In chemistry, saturation has six different meanings, all based on reaching a maximum capacity...
organic compound
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
s. Hydrogenation typically constitutes the addition of pairs of hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s to a molecule, generally an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
adds to double and triple bond
Triple bond
A triple bond in chemistry is a chemical bond between two chemical elements involving six bonding electrons instead of the usual two in a covalent single bond. The most common triple bond, that between two carbon atoms, can be found in alkynes. Other functional groups containing a triple bond are...
s in hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
s.
Because of the importance of hydrogen, many related reactions have been developed for its use. Most hydrogenations use gaseous hydrogen (H2), but some involve the alternative sources of hydrogen, not H2: these processes are called transfer hydrogenation
Transfer hydrogenation
Transfer hydrogenation is the addition of hydrogen to a molecule from a source other than gaseous H2. It is applied in industry and in organic synthesis, in part because of the inconvenience and expense of using gaseous H2...
s. The reverse reaction, removal of hydrogen from a molecule, is called dehydrogenation
Dehydrogenation
Dehydrogenation is a chemical reaction that involves the elimination of hydrogen . It is the reverse process of hydrogenation. Dehydrogenation reactions may be either large scale industrial processes or smaller scale laboratory procedures....
. A reaction where bonds are broken while hydrogen is added is called hydrogenolysis
Hydrogenolysis
Hydrogenolysis is a chemical reaction whereby a carbon–carbon or carbon–heteroatom single bond is cleaved or undergoes "lysis" by hydrogen. The heteroatom may vary, but it usually is oxygen, nitrogen, or sulfur. A related reaction is hydrogenation, where hydrogen is added to the molecule, without...
, a reaction that may occur to carbon-carbon and carbon-heteroatom (oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
, nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
or halogen
Halogen
The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
) bonds. Hydrogenation differs from protonation
Protonation
In chemistry, protonation is the addition of a proton to an atom, molecule, or ion. Some classic examples include*the protonation of water by sulfuric acid:*the protonation of isobutene in the formation of a carbocation:2C=CH2 + HBF4 → 3C+ + BF4−*the protonation of ammonia in the...
or hydride
Hydride
In chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties. In compounds that are regarded as hydrides, hydrogen is bonded to a more electropositive element or group...
addition: in hydrogenation, the products have the same charge as the reactants.
An illustrative example of a hydrogenation reaction is the addition of hydrogen to maleic acid
Maleic acid
Maleic acid is an organic compound that is a dicarboxylic acid, a molecule with two carboxyl groups. Maleic acid is the cis-isomer of butenedioic acid, whereas fumaric acid is the trans-isomer...
to form succinic acid
Succinic acid
Succinic acid is a dicarboxylic acid. Succinate plays a biochemical role in the citric acid cycle. The name derives from Latin succinum, meaning amber, from which the acid may be obtained....
. Numerous important applications of this petrochemical
Petrochemical
Petrochemicals are chemical products derived from petroleum. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as corn or sugar cane....
are found in pharmaceutical and food industries. Hydrogenation of unsaturated fat
Unsaturated fat
An unsaturated fat is a fat or fatty acid in which there is at least one double bond within the fatty acid chain. A fat molecule is monounsaturated if it contains one double bond, and polyunsaturated if it contains more than one double bond. Where double bonds are formed, hydrogen atoms are...
s produces saturated fat
Saturated fat
Saturated fat is fat that consists of triglycerides containing only saturated fatty acids. Saturated fatty acids have no double bonds between the individual carbon atoms of the fatty acid chain. That is, the chain of carbon atoms is fully "saturated" with hydrogen atoms...
s and, in some cases, trans fat
Trans fat
Trans fat is the common name for unsaturated fat with trans-isomer fatty acid. Because the term refers to the configuration of a double carbon-carbon bond, trans fats are sometimes monounsaturated or polyunsaturated, but never saturated....
s.
Process
Hydrogenation has three components, the unsaturatedSaturation (chemistry)
In chemistry, saturation has six different meanings, all based on reaching a maximum capacity...
substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst.
Substrate
The addition of H2 to an alkene affords an alkaneAlkane
Alkanes are chemical compounds that consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds without any cycles...
in the protypical reaction:
- RCH=CH2 + H2 → RCH2CH3 (R = alkyl, arylArylIn the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
)
Hydrogenation is sensitive to steric hindrance explaining the selectivity for reaction with the exocyclic double bond but not the internal double bond.
An important characteristic of alkene and alkyne hydrogenations, both the homogeneously and heterogeneously catalyzed versions, is that hydrogen addition occurs with "syn addition
Syn addition
In organic chemistry, syn and anti addition are different ways in which two substituents can be added to a double bond or triple bond. This article will use alkenes as examples....
", with hydrogen entering from the least hindered side. Typical substrates are listed in the table
| alkene Alkene In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond... , R2C=CR'2 |
alkane, R2CHCHR'2 | |
| alkyne Alkyne Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature... , RCCR |
alkene, cis-RHC=CHR' | |
| aldehyde Aldehyde An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group.... , RCHO |
primary alcohol, RCH2OH | |
| ketone Ketone In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology... , R2CO |
secondary alcohol, R2CHOH | |
| ester Ester Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and... , RCO2R' |
two alcohols, RCH2OH, R'OH | |
| imine Imine An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base... , RR'CNR" |
amine, RR'CHNHR" | |
| amide Amide In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an... , RC(O)NR'2 |
amine, RCH2NR'2 | |
| nitrile Nitrile A nitrile is any organic compound that has a -C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, one example being super glue .Inorganic compounds containing the -C≡N group are not called... , RCN |
imine, RHCNH | easily hydrogenated further |
| nitro Nitro compound Nitro compounds are organic compounds that contain one or more nitro functional groups . They are often highly explosive, especially when the compound contains more than one nitro group and is impure. The nitro group is one of the most common explosophores used globally... , RNO2 |
amine, RNH2 | |
Catalysts
With rare exceptions, no reaction below 480 °C (750 K or 900 °F) occurs between H2 and organic compounds in the absence of metal catalysts. The catalyst binds both the H2 and the unsaturated substrate and facilitates their union. platinumPlatinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
, palladium
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
, rhodium
Rhodium
Rhodium is a chemical element that is a rare, silvery-white, hard and chemically inert transition metal and a member of the platinum group. It has the chemical symbol Rh and atomic number 45. It is composed of only one isotope, 103Rh. Naturally occurring rhodium is found as the free metal, alloyed...
, and ruthenium
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
form highly active catalysts, which operate at lower temperatures and lower pressures of H2. Non-precious metal catalysts, especially those based on nickel (such as Raney nickel
Raney nickel
Raney nickel is a solid catalyst composed of fine grains of a nickel-aluminium alloy, used in many industrial processes. It was developed in 1926 by American]] engineer Murray Raney as an alternative catalyst for the hydrogenation of vegetable oils in industrial processes...
and Urushibara nickel) have also been developed as economical alternatives, but they are often slower or require higher temperatures. The trade-off is activity (speed of reaction) vs. cost of the catalyst and cost of the apparatus required for use of high pressures. Notice that the Raney-nickel catalysed hydrogenations require high pressures:
Two broad families of catalysts are known - homogeneous catalysts and heterogeneous catalysts. Homogeneous catalysts dissolve in the solvent that contains the unsaturated substrate. Heterogeneous catalysts are solids that are suspended in the same solvent with the substrate or are treated with gaseous substrate.
Homogeneous catalysts
Illustrative homogeneous catalysts include the rhodiumRhodium
Rhodium is a chemical element that is a rare, silvery-white, hard and chemically inert transition metal and a member of the platinum group. It has the chemical symbol Rh and atomic number 45. It is composed of only one isotope, 103Rh. Naturally occurring rhodium is found as the free metal, alloyed...
-based compound known as Wilkinson's catalyst
Wilkinson's catalyst
Wilkinson's catalyst is the common name for chlorotrisrhodium, a coordination compound with the formula RhCl3 . It is named after the late organometallic chemist and 1973 Nobel Laureate, Sir Geoffrey Wilkinson who popularized its use.-Structure and basic properties:The compound is a square planar,...
and the iridium
Iridium
Iridium is the chemical element with atomic number 77, and is represented by the symbol Ir. A very hard, brittle, silvery-white transition metal of the platinum family, iridium is the second-densest element and is the most corrosion-resistant metal, even at temperatures as high as 2000 °C...
-based Crabtree's catalyst
Crabtree's catalyst
Crabtree's catalyst is the name given to a complex of iridium with 1,5-cyclooctadiene, tris-cyclohexylphosphine, and pyridine. It is a homogeneous catalyst for hydrogenation reactions, developed by Robert H. Crabtree, a professor at Yale University...
. An example is the hydrogenation of carvone
Carvone
Carvone is a member of a family of chemicals called terpenoids. Carvone is found naturally in many essential oils, but is most abundant in the oils from seeds of caraway and dill.-Stereoisomerism and odor:...
:
Hydrogenation is sensitive to steric hindrance explaining the selectivity for reaction with the exocyclic double bond but not the internal double bond.
The activity and selectivity of homogeneous catalysts is adjusted by changing the ligands. For prochiral
Prochiral
In stereochemistry, prochiral molecules are those that can be converted from achiral to chiral in a single step.If two identical substituents are attached to a sp3-hybridized atom, the descriptors pro-R and pro-S are used to distinguish between the two...
substrates, the selectivity of the catalyst can be adjusted such that one enantiomeric product is favored. Asymmetric hydrogenation is also possible via heterogeneous catalysis on a metal that is modified by a chiral ligand.
Heterogeneous catalysts
Heterogeneous catalysts for hydrogenation are more common industrially. As in homogeneous catalysts, the activity is adjusted through changes in the environment around the metal, i.e. the coordination sphereCoordination sphere
In coordination chemistry, the coordination sphere refers to a central atom or ion and an array of molecules or anions, the ligands, around.Molecules that are attached noncovalently to the ligands are called the second coordination sphere....
. Different faces
Miller index
Miller indices form a notation system in crystallography for planes and directions in crystal lattices.In particular, a family of lattice planes is determined by three integers h, k, and ℓ, the Miller indices. They are written , and each index denotes a plane orthogonal to a direction in the...
of a crystalline heterogeneous catalyst display distinct activities, for example. Similarly, heterogeneous catalysts are affected by their supports, i.e. the material upon with the heterogeneous catalyst is bound.
In many cases, highly empirical modifications involve selective "poisons". Thus, a carefully chosen catalyst can be used to hydrogenate some functional groups without affecting others, such as the hydrogenation of alkenes without touching aromatic rings, or the selective hydrogenation of alkynes to alkenes using Lindlar's catalyst
Lindlar catalyst
A Lindlar catalyst is a heterogeneous catalyst that consists of palladium deposited on calcium carbonate and treated with various forms of lead. The lead additive serves to deactivate the palladium sites. A variety of "catalyst poisons" have been used including lead acetate and lead oxide. The...
. For example, when the catalyst palladium
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
is placed on barium sulfate
Barium sulfate
Barium sulfate is the inorganic compound with the chemical formula BaSO4. It is a white crystalline solid that is odorless and insoluble in water. It occurs as the mineral barite, which is the main commercial source of barium and materials prepared from it...
and then treated with quinoline
Quinoline
Quinoline is a heterocyclic aromatic organic compound. It has the formula C9H7N and is a colourless hygroscopic liquid with a strong odour. Aged samples, if exposed to light, become yellow and later brown...
, the resulting catalyst reduces alkynes only as far as alkenes. The Lindlar catalyst has been applied to the conversion of phenylacetylene
Phenylacetylene
Phenylacetylene is an alkyne hydrocarbon containing a phenyl group. It exists as a colorless, viscous liquid. In research, it is sometimes used as an analog for acetylene; being a liquid, it is easier to handle than acetylene gas.-Preparation:...
to styrene.
Asymmetric hydrogenation is also possible via heterogeneous catalysis on a metal that is modified by a chiral ligand.
Hydrogen sources
For hydrogenation, the obvious source of hydrogen is H2 gas itself, which is typically available commercially within the storage medium of a pressurized cylinder. The hydrogenation process often uses greater than 1 atmosphere of H2, usually conveyed from the cylinders and sometimes augmented by "booster pumps". Gaseous hydrogen is produced industrially from hydrocarbons by the process known as steam reformingSteam reforming
Fossil fuel reforming is a method of producing hydrogen or other useful products from fossil fuels such as natural gas. This is achieved in a processing device called a reformer which reacts steam at high temperature with the fossil fuel. The steam methane reformer is widely used in industry to...
.
Transfer hydrogenation
Hydrogen also can be extracted ("transferred") from "hydrogen-donors" in place of H2 gas. Hydrogen donors, which often serve as solventSolvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
s include hydrazine
Hydrazine
Hydrazine is an inorganic compound with the formula N2H4. It is a colourless flammable liquid with an ammonia-like odor. Hydrazine is highly toxic and dangerously unstable unless handled in solution. Approximately 260,000 tons are manufactured annually...
, dihydronaphthalene, dihydroanthracene, isopropanol, and formic acid
Formic acid
Formic acid is the simplest carboxylic acid. Its chemical formula is HCOOH or HCO2H. It is an important intermediate in chemical synthesis and occurs naturally, most notably in the venom of bee and ant stings. In fact, its name comes from the Latin word for ant, formica, referring to its early...
. In organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
, transfer hydrogenation
Transfer hydrogenation
Transfer hydrogenation is the addition of hydrogen to a molecule from a source other than gaseous H2. It is applied in industry and in organic synthesis, in part because of the inconvenience and expense of using gaseous H2...
is useful for the asymmetric reduction of polar unsaturated substrates, such as ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s, aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s, and imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
s.
Electrolytic hydrogenation
Polar substrates such as ketones can be hydrogenated electrochemically, using protic solvents and reducing equivalents as the source of hydrogen.Thermodynamics and mechanism
Hydrogenation is a strongly exothermicExothermic
In thermodynamics, the term exothermic describes a process or reaction that releases energy from the system, usually in the form of heat, but also in the form of light , electricity , or sound...
reaction. In the hydrogenation of vegetable oils and fatty acids, for example, the heat released is about 25 kcal per mole (105 kJ/mol), sufficient to raise the temperature of the oil by 1.6-1.7 °C per iodine number
Iodine number
The iodine value in chemistry is the mass of iodine in grams that is consumed by 100 grams of a chemical substance. An iodine solution is yellow/brown in color and any chemical group in the substance that reacts with iodine will make the color disappear at a precise concentration...
drop. The mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
of metal-catalyzed hydrogenation of alkenes and alkynes has been extensively studied. First of all isotope labeling using deuterium
Deuterium
Deuterium, also called heavy hydrogen, is one of two stable isotopes of hydrogen. It has a natural abundance in Earth's oceans of about one atom in of hydrogen . Deuterium accounts for approximately 0.0156% of all naturally occurring hydrogen in Earth's oceans, while the most common isotope ...
confirms the regiochemistry of the addition:
- RCH=CH2 + D2 → RCHDCH2D
Heterogeneous catalysis
On solids, the accepted mechanism today is called the Horiuti-Polanyi mechanism.- Binding of the unsaturated bond, and hydrogen dissociation into atomic hydrogen onto the catalyst
- Addition of one atom of hydrogen; this step is reversible
- Addition of the second atom; effectively irreversible under hydrogenating conditions.
In the second step, the metallointermediate formed is a saturated compound that can rotate and then break down, again detaching the alkene from the catalyst. Consequently, contact with a hydrogenation catalyst necessarily causes cis-trans-isomerization. This is a problem in partial hydrogenation, while in complete hydrogenation the produced trans-alkene is eventually hydrogenated.
For aromatic substrates, the first bond is hardest to hydrogenate because of the free energy penalty for breaking the aromatic system. The product of this is a cyclohexadiene, which is extremely active and cannot be isolated; in conditions reducing enough to break the aromatization, it is immediately reduced to a cyclohexene. The cyclohexene
Cyclohexene
Cyclohexene is a hydrocarbon with the formula C6H10. This cycloalkene is a colorless liquid with a sharp smell. It is an intermediate in various industrial processes...
is ordinarily reduced immediately to a fully saturated cyclohexane, but special modifications to the catalysts (such as the use of the anti-solvent water on ruthenium) can preserve some of the cyclohexene, if that is a desired product.
Homogeneous catalysis
In many homogeneous hydrogenation processes, the metal binds to both components to give an intermediate alkene-metal(H)2 complex. The general sequence of reactions is assumed to be as follows or a related sequence of steps:- binding of the hydrogen to give a dihydride complex ("oxidative addition"):
- LnM + H2 → LnMH2
- binding of alkene:
- LnM(η2H2) + CH2=CHR → Ln-1MH2(CH2=CHR) + L
- transfer of one hydrogen atom from the metal to carbon (migratory insertion)
- Ln-1MH2(CH2=CHR) → Ln-1M(H)(CH2-CH2R)
- transfer of the second hydrogen atom from the metal to the alkyl group with simultaneous dissociation of the alkane ("reductive elimination")
- Ln-1M(H)(CH2-CH2R) → Ln-1M + CH3-CH2R
Preceding the oxidative addition of H2 is the formation of a dihydrogen complex
Dihydrogen complex
Dihydrogen complexes are coordination complexes containing intact H2 as a ligand. The prototypical complex is W32. This class of compounds represent intermediates in metal-catalyzed reactions involving hydrogen. Hundreds of dihydrogen complexes have been reported...
.
Inorganic substrates
The hydrogenation of nitrogen to give ammonia is conducted on a vast scale by the Haber-Bosch process, consuming an estimated 1% of the world's energy supply.Oxygen can be partially hydrogenated to give hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
, although this process has not been commercialized.
Industrial applications
Catalytic hydrogenation has diverse industrial uses.In petrochemical processes, hydrogenation is used to convert alkenes and aromatics into saturated alkanes (paraffins) and cycloalkanes (naphthenes), which are less toxic and less reactive. For example, mineral turpentine
Mineral turpentine
Mineral turpentine, also known as turpentine substitute, turps substitute, or just turps is an inexpensive petroleum-based replacement for the vegetable-based turpentine...
is usually hydrogenated. Hydrocracking of heavy residues into diesel is another application. In isomerization and catalytic reforming
Catalytic reforming
Catalytic reforming is a chemical process used to convert petroleum refinery naphthas, typically having low octane ratings, into high-octane liquid products called reformates which are components of high-octane gasoline...
processes, some hydrogen pressure is maintained to hydrogenolyze
Hydrogenolysis
Hydrogenolysis is a chemical reaction whereby a carbon–carbon or carbon–heteroatom single bond is cleaved or undergoes "lysis" by hydrogen. The heteroatom may vary, but it usually is oxygen, nitrogen, or sulfur. A related reaction is hydrogenation, where hydrogen is added to the molecule, without...
coke
Coke
Coke may refer to:* Coca-Cola, a soft drink originally based on coca leaf extract** The Coca-Cola Company, makers of this drink** Cola, any soft drink similar to Coca-Cola** Soft drink, any non-alcoholic carbonated beverage* Coca, a plant...
formed on the catalyst and prevent its accumulation.
Xylitol
Xylitol
Xylitol is a sugar alcohol sweetener used as a naturally occurring sugar substitute. It is found in the fibers of many fruits and vegetables, and can be extracted from various berries, oats, and mushrooms, as well as fibrous material such as corn husks and sugar cane bagasse, and birch...
, a polyol
Polyol
A polyol is an alcohol containing multiple hydroxyl groups. In two technological disciplines the term "polyol" has a special meaning: food science and polymer chemistry.- Polyols in food science :...
, is produced by hydrogenation of the sugar xylose
Xylose
Xylose is a sugar first isolated from wood, and named for it. Xylose is classified as a monosaccharide of the aldopentose type, which means that it contains five carbon atoms and includes an aldehyde functional group. It is the precursor to hemicellulose, one of the main constituents of biomass...
, an aldehyde.
In the food industry
Hydrogenation is widely applied to the processing of vegetable oils and fatFat
Fats consist of a wide group of compounds that are generally soluble in organic solvents and generally insoluble in water. Chemically, fats are triglycerides, triesters of glycerol and any of several fatty acids. Fats may be either solid or liquid at room temperature, depending on their structure...
s. Complete hydrogenation converts unsaturated fatty acid
Fatty acid
In chemistry, especially biochemistry, a fatty acid is a carboxylic acid with a long unbranched aliphatic tail , which is either saturated or unsaturated. Most naturally occurring fatty acids have a chain of an even number of carbon atoms, from 4 to 28. Fatty acids are usually derived from...
s to saturated
Saturated fat
Saturated fat is fat that consists of triglycerides containing only saturated fatty acids. Saturated fatty acids have no double bonds between the individual carbon atoms of the fatty acid chain. That is, the chain of carbon atoms is fully "saturated" with hydrogen atoms...
ones. In practice the process is not usually carried to completion. Since the original oils usually contain more than one carbon-carbon double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
per molecule (that is, they are polyunsaturated), the result is usually described as partially hydrogenated vegetable oil; that is some, but usually not all, of the carbon-carbon double bonds in each molecule have been reduced. This is done by restricting the amount of hydrogen (or reducing agent) allowed to react with the fat.
Hydrogenation results in the conversion of liquid vegetable oils to solid or semi-solid fats, such as those present in margarine
Margarine
Margarine , as a generic term, can indicate any of a wide range of butter substitutes, typically composed of vegetable oils. In many parts of the world, the market share of margarine and spreads has overtaken that of butter...
. Changing the degree of saturation of the fat changes some important physical properties such as the melting range, which is why liquid oils become semi-solid. Solid or semi-solid fats are preferred for baking because the way the fat mixes with flour produces a more desirable texture in the baked product. Because partially hydrogenated vegetable oils are cheaper than animal source fats, they are available in a wide range of consistencies, and have other desirable characteristics (e.g., increased oxidative stability/longer shelf life), they are the predominant fats used as shortening
Shortening
Shortening is any fat that is solid at room temperature and used to make crumbly pastry. The reason it is called shortening is because it prevents cross-linkage between gluten molecules. Cross linking is what causes doughs to be sticky. Seeing as cake is not meant to be sticky, shortening is used...
in most commercial baked goods.
Health implications
A side effect of incomplete hydrogenation having implications for human health is the isomerization of some of the remaining unsaturated carbon bonds. The cis configuration of these double bondDouble bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
s predominates in the unprocessed fats in most edible fat sources, but incomplete hydrogenation partially converts these molecules to trans isomers, which have been implicated in circulatory diseases including heart disease
Heart disease
Heart disease, cardiac disease or cardiopathy is an umbrella term for a variety of diseases affecting the heart. , it is the leading cause of death in the United States, England, Canada and Wales, accounting for 25.4% of the total deaths in the United States.-Types:-Coronary heart disease:Coronary...
(see trans fat
Trans fat
Trans fat is the common name for unsaturated fat with trans-isomer fatty acid. Because the term refers to the configuration of a double carbon-carbon bond, trans fats are sometimes monounsaturated or polyunsaturated, but never saturated....
s). The conversion from cis to trans bonds is favored because the trans configuration has lower energy than the natural cis one. At equilibrium, the trans/cis isomer ratio is about 2:1. Food legislation in the US and codes of practice in EU have long required labels declaring the fat content of foods in retail trade and, more recently, have also required declaration of the trans fat content. Trans fats are banned in Denmark
Denmark
Denmark is a Scandinavian country in Northern Europe. The countries of Denmark and Greenland, as well as the Faroe Islands, constitute the Kingdom of Denmark . It is the southernmost of the Nordic countries, southwest of Sweden and south of Norway, and bordered to the south by Germany. Denmark...
and New York City
New York City
New York is the most populous city in the United States and the center of the New York Metropolitan Area, one of the most populous metropolitan areas in the world. New York exerts a significant impact upon global commerce, finance, media, art, fashion, research, technology, education, and...
.
History
The earliest hydrogenation is that of platinumPlatinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
catalyzed
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
addition of hydrogen to oxygen in the Döbereiner's lamp
Döbereiner's lamp
Döbereiner's lamp is a lighter invented in 1823 by the German chemist Johann Wolfgang Döbereiner, the lighter is based on the Fürstenberger lighter and was in production until ca. 1880. In the jar, zinc metal reacts with sulfuric acid to produce hydrogen gas. When a valve is opened, a jet of...
, a device commercialized as early as 1823. The French chemist Paul Sabatier
Paul Sabatier (chemist)
Paul Sabatier FRS was a French chemist, born at Carcassonne. He taught science classes most of his life before he became Dean of the Faculty of Science at the University of Toulouse in 1905....
is considered the father of the hydrogenation process. In 1897, building on the earlier work of James Boyce
James F. Boyce, Sr.
James F. Boyce was an American chemist of the late 19th and early 20th centuries involved in the manufacturing of soaps and detergents. He also pioneered techniques now used in the isolation and removal of consumable hydrogenated vegetable oils from plants, especially cottonseed...
, an American chemist working in the manufacture of soap products, he discovered that the introduction of a trace of nickel as a catalyst facilitated the addition of hydrogen to molecules of gaseous hydrocarbons in what is now known as the Sabatier process. For this work Sabatier shared the 1912 Nobel Prize in Chemistry
Nobel Prize in Chemistry
The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outstanding contributions in chemistry, physics, literature,...
. Wilhelm Normann
Wilhelm Normann
Wilhelm Normann was a German chemist who introduced the hydrogenation of fats in 1901, creating what later became known as trans fats...
was awarded a patent in Germany in 1902 and in Britain in 1903 for the hydrogenation of liquid oils, which was the beginning of what is now a world wide industry. The commercially important Haber-Bosch process, first described in 1905, involves hydrogenation of nitrogen. In the Fischer-Tropsch process
Fischer-Tropsch process
The Fischer–Tropsch process is a set of chemical reactions that convert a mixture of carbon monoxide and hydrogen into liquid hydrocarbons. The process, a key component of gas to liquids technology, produces a petroleum substitute, typically from coal, natural gas, or biomass for use as synthetic...
, reported in 1922 carbon monoxide, which is easily derived from coal, is hydrogenated to liquid fuels.
Also in 1922, Voorhees and Adams described an apparatus for performing hydrogenation under pressures above one atmosphere. The Parr shaker, the first product to allow hydrogenation using elevated pressures and temperatures, was commercialized in 1926 based on Voorhees and Adams’ research and remains in widespread use. In 1924 Murray Raney
Murray Raney
Murray Raney was an American mechanical engineer born in Carrollton, Kentucky. He was the developer of a nickel catalyst that became known as Raney nickel, which is often used in industrial processes and scientific research for the hydrogenation of multiple covalent bonds present in...
developed a nickel fine powder catalyst named after him which is still widely used in hydrogenation reactions such as conversion of nitriles to amines or the production of margarine. In 1938, Otto Roelen
Otto Roelen
Otto Roelen was a German chemist.Roelen studied chemistry and graduated in 1922 from Technische Hochschule Stuttgart. He worked with Franz Fischer and Hans Tropsch at the Kaiser Wilhelm Institute for Coal Research from 1922...
described the oxo process which involves the addition of both hydrogen and carbon monoxide to alkenes, giving aldehydes. Since this process entails C-C coupling, it and its many variations (see carbonylation
Carbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry.-Organic chemistry:...
) remains highly topical into the new decade. The 1960s witnessed the development of homogeneous catalysts, e.g., Wilkinson's catalyst
Wilkinson's catalyst
Wilkinson's catalyst is the common name for chlorotrisrhodium, a coordination compound with the formula RhCl3 . It is named after the late organometallic chemist and 1973 Nobel Laureate, Sir Geoffrey Wilkinson who popularized its use.-Structure and basic properties:The compound is a square planar,...
. In the 1980s, the Noyori asymmetric hydrogenation
Noyori asymmetric hydrogenation
The Noyori asymmetric hydrogenation is a chemical reaction described as an asymmetric reduction of β-keto-esters.Both enantiomers of BINAP are commercially available and widely used...
represented one of the first applications of hydrogenation in asymmetric synthesis, a growing application in the production of fine chemicals.
Metal-free hydrogenation
For all practical purposes, hydrogenation requires a metal catalyst. Hydrogenation can, however, proceed from some hydrogen donors without catalysts, illustrative hydrogen donors being diimide and aluminium isopropoxideAluminium isopropoxide
Aluminium isopropoxide is the chemical compound usually described with the formula Al3, where i-Pr is the isopropyl group . This colourless solid is a useful reagent in organic synthesis...
. Some metal-free catalytic systems have been investigated in academic research. One such system for reduction of ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s consists of tert-butanol
Tert-Butanol
tert-Butanol, or 2-methyl-2-propanol, is the simplest tertiary alcohol. It is one of the four isomers of butanol. tert-Butanol is a clear liquid with a camphor-like odor. It is very soluble in water and miscible with ethanol and diethyl ether...
and potassium tert-butoxide
Potassium tert-butoxide
Potassium tert-butoxide is the chemical compound with the formula 3COK. This colourless solid is a strong base useful in organic synthesis. It exists as a tetrameric cubane-like cluster...
and very high temperatures. The reaction depicted below describes the hydrogenation of benzophenone
Benzophenone
Benzophenone is the organic compound with the formula 2CO, generally abbreviated Ph2CO. Benzophenone is a widely used building block in organic chemistry, being the parent diarylketone.-Uses:...
:
A chemical kinetics
Chemical kinetics
Chemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
study found this reaction is first-order
Order of reaction
In chemical kinetics, the order of reaction with respect to certain reactant, is defined as the power to which its concentration term in the rate equation is raised .For example, given a chemical reaction 2A + B → C with a rate equation...
in all three reactants suggesting a cyclic 6-membered transition state
Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
.
Another system for metal-free hydrogenation is based on the phosphine
Phosphine
Phosphine is the compound with the chemical formula PH3. It is a colorless, flammable, toxic gas. Pure phosphine is odourless, but technical grade samples have a highly unpleasant odor like garlic or rotting fish, due to the presence of substituted phosphine and diphosphine...
-borane
Borane
In chemistry, a borane is a chemical compound of boron and hydrogen. The boranes comprise a large group of compounds with the generic formulae of BxHy. These compounds do not occur in nature. Many of the boranes readily oxidise on contact with air, some violently. The parent member BH3 is called...
, compound 1, which has been called a frustrated Lewis pair
Frustrated Lewis pair
In chemistry, a frustrated Lewis pair is a compound or mixture containing a Lewis acid and a Lewis base that, because of steric hindrance, cannot combine to form an adduct...
. It reversibly accepts dihydrogen at relatively low temperatures to form the phosphonium
Phosphonium
The phosphonium cation describes positively charged polyatomic cations with the chemical formula . Salts of the parent PH4+ are rarely encountered, but this ion is an intermediate in the preparation of the industrially useful tetrakisphosphonium chloride:Organic phosphonium salts are common...
borate
Borate
Borates are chemical compounds which contain oxoanions of boron in oxidation state +3. The simplest borate ion, BO33−, has a trigonal planar structure. Other borates are made up of trigonal BO3 or tetrahedral BO4 structural units, sharing oxygen atoms...
2 which can reduce simple hindered imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
s.
The reduction of nitrobenzene
Nitrobenzene
Nitrobenzene is an organic compound with the chemical formula C6H5NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale as a precursor to aniline. Although occasionally used as a flavoring or perfume...
to aniline
Aniline
Aniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Being a precursor to many industrial chemicals, its main use is in the manufacture of precursors to polyurethane...
has been reported to be catalysed by fullerene
Fullerene
A fullerene is any molecule composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Spherical fullerenes are also called buckyballs, and they resemble the balls used in association football. Cylindrical ones are called carbon nanotubes or buckytubes...
, its mono-anion, atmospheric hydrogen and UV light.
Equipment used for hydrogenation
Today’s bench chemist has three main choices of hydrogenation equipment:- Batch hydrogenation under atmospheric conditions
- Batch hydrogenation at elevated temperature and/or pressure
- Flow hydrogenation
Batch hydrogenation under atmospheric conditions
The original and still a commonly practised form of hydrogenation in teaching laboratories, this process is usually effected by adding solid catalyst to a round bottom flask of dissolved reactant which has been evacuated using nitrogenNitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
or argon
Argon
Argon is a chemical element represented by the symbol Ar. Argon has atomic number 18 and is the third element in group 18 of the periodic table . Argon is the third most common gas in the Earth's atmosphere, at 0.93%, making it more common than carbon dioxide...
gas and sealing the mixture with a penetrable rubber seal. Hydrogen gas is then supplied from a H2-filled balloon
Balloon
A balloon is an inflatable flexible bag filled with a gas, such as helium, hydrogen, nitrous oxide, oxygen, or air. Modern balloons can be made from materials such as rubber, latex, polychloroprene, or a nylon fabric, while some early balloons were made of dried animal bladders, such as the pig...
. The resulting three phase mixture is agitated to promote mixing. Hydrogen uptake can be monitored, which can be useful for monitoring progress of a hydrogenation. This is achieved by either using a graduated tube containing a coloured liquid, usually aqueous copper sulfate or with gauges
Sight glass
A sight glass or water gauge is a transparent tube through which the operator of a tank or boiler can observe the level of liquid contained within.-Liquid in tanks:...
for each reaction vessel.
Batch hydrogenation at elevated temperature and/or pressure
Since many hydrogenation reactions such as hydrogenolysisHydrogenolysis
Hydrogenolysis is a chemical reaction whereby a carbon–carbon or carbon–heteroatom single bond is cleaved or undergoes "lysis" by hydrogen. The heteroatom may vary, but it usually is oxygen, nitrogen, or sulfur. A related reaction is hydrogenation, where hydrogen is added to the molecule, without...
of protecting groups and the reduction of aromatic systems proceed extremely sluggishly at atmospheric temperature and pressure, pressurised systems are popular. In these cases, catalyst is added to a solution of reactant under an inert atmosphere in a pressure vessel
Pressure vessel
A pressure vessel is a closed container designed to hold gases or liquids at a pressure substantially different from the ambient pressure.The pressure differential is dangerous and many fatal accidents have occurred in the history of their development and operation. Consequently, their design,...
. Hydrogen is added directly from a cylinder or built in laboratory hydrogen source, and the pressurized slurry is mechanically rocked to provide agitation or a spinning basket is used. Heat may also be used, as the pressure compensates for the associated reduction in gas solubility.
Flow hydrogenation
Flow hydrogenation has become a popular technique at the bench and increasingly the process scale. This technique involves continuously flowing a dilute stream of dissolved reactant over a fixed bed catalyst in the presence of hydrogen. Using established HPLCHigh-performance liquid chromatography
High-performance liquid chromatography , HPLC, is a chromatographic technique that can separate a mixture of compounds and is used in biochemistry and analytical chemistry to identify, quantify and purify the individual components of the mixture.HPLC typically utilizes different types of stationary...
technology, this technique allows the application of pressures from atmospheric to 1,450 PSI. Elevated temperatures may also be used. At the bench scale, systems use a range of pre-packed catalysts which eliminates the need for weighing and filtering pyrophoric catalysts.
Industrial reactors
Catalytic hydrogenation is done in a tubular plug-flow reactor (PFR) packed with a supported catalyst. The pressures and temperatures are typically high, although this depends on the catalyst. Catalyst loading is typically much lower than in laboratory batch hydrogenation, and various promoters are added to the metal, or mixed metals are used, to improve activity, selectivity and catalyst stability. The use of nickel is common despite its low activity, due to its low cost compared to precious metals.Gas Liquid Induction Reactors (Hydrogenator) are also used for carrying out catalytic hydrogenation.
See also
- DehydrogenationDehydrogenationDehydrogenation is a chemical reaction that involves the elimination of hydrogen . It is the reverse process of hydrogenation. Dehydrogenation reactions may be either large scale industrial processes or smaller scale laboratory procedures....
- Transfer hydrogenationTransfer hydrogenationTransfer hydrogenation is the addition of hydrogen to a molecule from a source other than gaseous H2. It is applied in industry and in organic synthesis, in part because of the inconvenience and expense of using gaseous H2...
- HydrogenolysisHydrogenolysisHydrogenolysis is a chemical reaction whereby a carbon–carbon or carbon–heteroatom single bond is cleaved or undergoes "lysis" by hydrogen. The heteroatom may vary, but it usually is oxygen, nitrogen, or sulfur. A related reaction is hydrogenation, where hydrogen is added to the molecule, without...
- HydrodesulfurizationHydrodesulfurizationHydrodesulfurization is a catalytic chemical process widely used to remove sulfur from natural gas and from refined petroleum products such as gasoline or petrol, jet fuel, kerosene, diesel fuel, and fuel oils...
, Hydrotreater and Oil desulfurization - Timeline of hydrogen technologiesTimeline of hydrogen technologiesTimeline of hydrogen technologies — A timeline of the history of hydrogen technology.-1600s:* 1625 - First description of hydrogen by Johann Baptista van Helmont...
Further reading
- examples of hydrogenation from Organic Syntheses:
- early work on transfer hydrogenation: Davies, R. R.; Hodgson, H. H. J. Chem. Soc. 1943, 281. Leggether, B. E.; Brown, R. K. Can. J. Chem. 1960, 38, 2363. Kuhn, L. P. J. Am. Chem. Soc. 1951, 73, 1510.
External Links
- "The Magic of Hydro" Popular Mechanics, June 1931, pp. 107-109 early article for the general public on hydrogenation of oil produces in the 1930s