
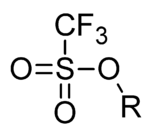
Triflate
Encyclopedia
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
with the formula CF3SO3-. The triflate group is often represented by -OTf, as opposed to -Tf (triflyl). For example, the n-butyl
Butyl
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula -C4H9, derived from either of the two isomers of butane....
triflate, CH3CH2CH2CH2OTf.
The triflate anion, CF3SO3- is an extremely stable polyatomic ion
Polyatomic ion
A polyatomic ion, also known as a molecular ion, is a charged species composed of two or more atoms covalently bonded or of a metal complex that can be considered as acting as a single unit in the context of acid and base chemistry or in the formation of salts. The prefix "poly-" means "many," in...
, being the conjugate base
Conjugate acid
Within the Brønsted–Lowry acid-base theory , a conjugate acid is the acid member, HX, of a pair of two compounds that transform into each other by gain or loss of a proton. A conjugate acid can also be seen as the chemical substance that releases, or donates, a proton in the forward chemical...
of triflic acid (CF3SO3H), one of the strongest acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
s known. It is defined as a superacid
Superacid
According to the classical definition superacid is an acid with an acidity greater than that of 100% pure sulfuric acid, which has a Hammett acidity function of −12. According to the modern definition, superacid is a medium, in which the chemical potential of the proton is higher than in pure...
, because it is more acidic than pure sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
.
Applications
A triflate group is an excellent leaving groupLeaving group
In chemistry, a leaving group is a molecular fragment that departs with a pair of electrons in heterolytic bond cleavage. Leaving groups can be anions or neutral molecules. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters, such as para-toluenesulfonate...
used in certain organic reactions
Organic Reactions
Organic Reactions is a secondary reference which synthesizes the organic chemistry literature around particular chemical transformations. Each chapter of Organic Reactions is devoted to a particular organic chemical reaction, and chapters provide exhaustive coverage of literature work in the form...
such as nucleophilic substitution
Nucleophilic substitution
In organic and inorganic chemistry, nucleophilic substitution is a fundamental class of reactions in which an electron nucleophile selectively bonds with or attacks the positive or partially positive charge of an atom or a group of atoms called the leaving group; the positive or partially positive...
, Suzuki couplings and Heck reaction
Heck reaction
The Heck reaction is the chemical reaction of an unsaturated halide with an alkene and a base and palladium catalyst to form a substituted alkene. Together with the other palladium-catalyzed cross-coupling reactions, this reaction is of great importance, as it allows one to do substitution...
s. Since alkyl triflates are extremely reactive in SN2 reactions
SN2 reaction
The SN2 reaction is a type of nucleophilic substitution, where a lone pair from a nucleophile attacks an electron deficient electrophilic center and bonds to it, expelling another group called a leaving group. Thus the incoming group replaces the leaving group in one step...
, they must be stored in conditions free of nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
s (such as water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
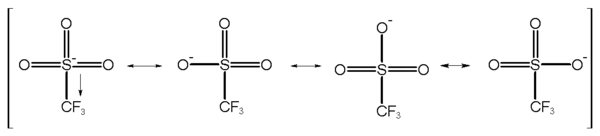
). The anion owes its stability to resonance stabilization which causes the negative charge to be spread over the three oxygen atoms and the sulfur atom. An additional stabilization is achieved by the trifluoromethyl
Trifluoromethyl
Trifluoromethyl is a functional group in organofluorines that has the formula -CF3. The naming of is group is derived from the methyl group , by replacing each hydrogen atom by a fluorine atom. The trifluomethyl group has a significant electronegativity that is often described as being...
group as a strong electron-withdrawing group.
Triflates have also been applied as ligands for group 11
Group 11 element
A Group 11 element is one in the series of elements in group 11 in the periodic table, consisting of transition metals which are the traditional coinage metals of copper , silver , and gold...
and 13 metals along with lanthanide
Lanthanide
The lanthanide or lanthanoid series comprises the fifteen metallic chemical elements with atomic numbers 57 through 71, from lanthanum through lutetium...
s.
Lithium triflates are used in some lithium ion batteries as a component of the electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
.
A mild triflating reagent is phenyl triflimide or N,N-bis(trifluoromethylsulfonyl)aniline, where the by-product is [CF3SO2N-Ph]-.
Triflate salts
Triflate salts are thermally very stable with melting points up to 350°C for sodiumSodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
, boron
Boron
Boron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the...
and silver
Silver
Silver is a metallic chemical element with the chemical symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal...
salts especially in water-free form. They can be obtained directly from triflic acid and the metal hydroxide or metal carbonate in water. Alternatively, they can be obtained from reacting metal chlorides with neat triflic acid or silver triflate, or from reacting barium triflate with metal sulfates in water:
- MCln + n HOTf → M(OTf)n + n HCl
- MCln + n AgOTf → M(OTf)n + n AgCl ↓
- M(SO4)n + n Ba(OTf)2 → M(OTf)2n + n BaSO4 ↓
Triflates are used as Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
s in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
because of their stability compared to more traditional catalysts unstable in water such as aluminium chloride
Aluminium chloride
Aluminium chloride is the main compound of aluminium and chlorine. It is white, but samples are often contaminated with iron trichloride, giving it a yellow colour. The solid has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium metal, but large...
. Especially useful are the lanthanide triflates
Lanthanide triflates
Lanthanide triflates are triflate salts of the lanthanide family with many uses in organic chemistry as Lewis acid catalysts. The catalysts act similarly to aluminium chloride or ferric chloride, but are stable in water, which makes it possible to use water as a solvent instead of organic...
of the type Ln(OTf)3 (where Ln = La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Y). Another popular catalyst scandium triflate is used in such reactions as aldol reaction
Aldol reaction
The aldol reaction is a powerful means of forming carbon–carbon bonds in organic chemistry.Discovered independently by Charles-Adolphe Wurtz and Alexander Porfyrevich Borodin in 1872, the reaction combines two carbonyl compounds to form a new β-hydroxy carbonyl compound...
s and Diels-Alder reaction
Diels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
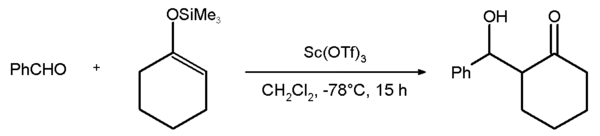
s. An example is the Mukaiyama aldol addition
Mukaiyama aldol addition
The Mukaiyama aldol addition is an organic reaction and a type of aldol reaction between a silyl enol ether and an aldehyde catalyzed by a Lewis acid. This choice of reactants allows for a crossed aldol reaction between an aldehyde and a ketone or a different aldehyde without self-condensation of...
reaction between benzaldehyde
Benzaldehyde
Benzaldehyde is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful. This colorless liquid has a characteristic pleasant almond-like odor...
and the silyl enol ether
Silyl enol ether
Silyl enol ethers in organic chemistry are a class of organic compounds that share a common functional group composed of an enolate bonded through its oxygen terminus to an organosilicon group....
of cyclohexanone
Cyclohexanone
Cyclohexanone is the organic compound with the formula 5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oil has an odor reminiscent of peardrop sweets as well as acetone. Over time, samples assume a yellow color due to oxidation...
with an 81% chemical yield. The corresponding reaction with the yttrium
Yttrium
Yttrium is a chemical element with symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and it has often been classified as a "rare earth element". Yttrium is almost always found combined with the lanthanides in rare earth minerals and is...
salt fails:
See also
- methyl triflateMethyl triflateMethyl trifluoromethanesulfonate, is commonly called methyl triflate, and has the chemical formula CF3SO2-OCH3, and is used as a very powerful methylating reagent in chemistry....
- nonaflateNonaflateNonaflate, CF3CF2CF2CF2SO3– is the common name given to nonafluorobutanesulfonates, the salts or esters of perfluorobutanesulfonic acid. Its uses are similar to those of triflate....
- trifluoromethanesulfonic acidTrifluoromethanesulfonic acidTrifluoromethanesulfonic acid, also known as triflic acid, TFMS, TFSA, HOTf or TfOH, is a sulfonic acid with the chemical formula CF3SO3H. It is one of the strongest acids. Triflic acid is mainly used in research as a catalyst for esterification.-Properties:Triflic acid is a hygroscopic, colorless...
- metal triflimidateMetal triflimidateA metal triflimidate Mn in organic chemistry is a metal salt of triflimidic acid and used as a catalyst .metal Triflimidates are prepared by reaction of metal oxides, carbonates, hydroxides, or halides with triflimidic acid in water as the hydrate MTfn.xH20 with x ranging from zero to nine...

