_iodide.gif)
Samarium(II) iodide
Encyclopedia
Samarium iodide (SmI2, also known as "Kagan reagent") is a green solid composed of samarium
and iodine
, with a melting point of 520 °C where the samarium atom has a coordination number
of seven in a capped octahedral configuration. It can be formed by high temperature decomposition
of SmI3 (the more stable iodide), but a convenient lab preparation is to react Sm powder with 1,2-diiodoethane in anhydrous THF
, or CH2I2 may also be used. Samarium(II) iodide is a powerful reducing agent
– for example it rapidly reduces water
to hydrogen
. It is available commercially as a dark blue 0.1 M
solution in THF.
formation, for example in a Barbier reaction
(similar to the Grignard reaction
) between a ketone
and an alkyl iodide to form a tertiary alcohol:
 Typical reaction conditions use SmI2 in THF in the presence of catalytic NiI2.
Typical reaction conditions use SmI2 in THF in the presence of catalytic NiI2.
Ester
s react similarly (adding two R groups), but aldehyde
s give by-products. The reaction is convenient in that it is often very rapid (5 minutes or less in the cold). Although samarium(II) iodide is considered a powerful single-electron reducing agent, it does display remarkable chemoselectivity
among functional groups. For example, sulfone
s and sulfoxide
s can be reduced to the corresponding sulfide
in the presence of a variety of carbonyl
-containing functionalities (such as ester
s, ketone
s, amide
s, aldehyde
s, etc.). This is presumably due to the considerably slower reaction with carbonyl
s as compared to sulfone
s and sulfoxide
s. Furthermore, hydrodehalogenation of halogenated hydrocarbons to the corresponding hydrocarbon
compound can be achieved using samarium(II) iodide. Also, it can be monitored by the color change that occurs as the dark blue color of SmI2 in THF discharges to a light yellow once the reaction has occurred. The picture shows the dark colour disappearing immediately upon contact with the Barbier reaction
mixture.
Work-up is with dilute hydrochloric acid
, and the samarium is removed as aqueous Sm3+.
Carbonyl compounds can also be coupled with simple alkenes to form five, six or eight membered rings.
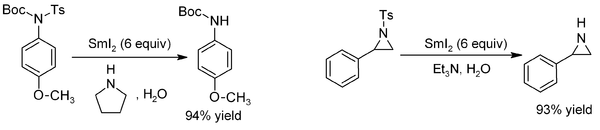
Tosyl
groups can be removed from N-tosylamides almost instantaneously, using SmI2 in conjunction with a base. The reaction is even effective for the synthesis of sensitive amine
s such as aziridine
s:
In the Markó-Lam deoxygenation
, an alcohol could be almost instantaneously deoxygenated by reducing their toluate ester in presence of SmI2.
The applications of SmI2 have been reviewed.
Samarium
Samarium is a chemical element with the symbol Sm, atomic number 62 and atomic weight 150.36. It is a moderately hard silvery metal which readily oxidizes in air. Being a typical member of the lanthanide series, samarium usually assumes the oxidation state +3...
and iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
, with a melting point of 520 °C where the samarium atom has a coordination number
Coordination number
In chemistry and crystallography, the coordination number of a central atom in a molecule or crystal is the number of its nearest neighbours. This number is determined somewhat differently for molecules and for crystals....
of seven in a capped octahedral configuration. It can be formed by high temperature decomposition
Chemical decomposition
Chemical decomposition, analysis or breakdown is the separation of a chemical compound into elements or simpler compounds. It is sometimes defined as the exact opposite of a chemical synthesis. Chemical decomposition is often an undesired chemical reaction...
of SmI3 (the more stable iodide), but a convenient lab preparation is to react Sm powder with 1,2-diiodoethane in anhydrous THF
ThF
Follicular B helper T cells , are antigen-experienced CD4+ T cells found in the B cell follicles of secondary lymphoid organs such as lymph nodes, spleens and Peyer's patches, and are identified by their constitutive expression of the B cell follicle homing receptor CXCR5...
, or CH2I2 may also be used. Samarium(II) iodide is a powerful reducing agent
Reducing agent
A reducing agent is the element or compound in a reduction-oxidation reaction that donates an electron to another species; however, since the reducer loses an electron we say it is "oxidized"...
– for example it rapidly reduces water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
to hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
. It is available commercially as a dark blue 0.1 M
Concentration
In chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
solution in THF.
- Sm + ICH2CH2I → SmI2 + C2H4
Reactions
Samarium(II) iodide has become a popular reagent for carbon-carbon bondCarbon-carbon bond
A carbon–carbon bond is a covalent bond between two carbon atoms. The most common form is the single bond: a bond composed of two electrons, one from each of the two atoms. The carbon–carbon single bond is a sigma bond and is said to be formed between one hybridized orbital from each...
formation, for example in a Barbier reaction
Barbier reaction
The Barbier reaction is an organic reaction between an alkyl halide and a carbonyl group as an electrophilic substrate in the presence of magnesium, aluminium, zinc, indium, tin or its salts. The reaction product is a primary, secondary or tertiary alcohol...
(similar to the Grignard reaction
Grignard reaction
The Grignard reaction is an organometallic chemical reaction in which alkyl- or aryl-magnesium halides add to a carbonyl group in an aldehyde or ketone. This reaction is an important tool for the formation of carbon–carbon bonds...
) between a ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
and an alkyl iodide to form a tertiary alcohol:
- R1I + R2COR3 → R1R2C(OH)R3

Ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
s react similarly (adding two R groups), but aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s give by-products. The reaction is convenient in that it is often very rapid (5 minutes or less in the cold). Although samarium(II) iodide is considered a powerful single-electron reducing agent, it does display remarkable chemoselectivity
Chemoselectivity
Chemical reactions are defined usually in small contexts , such generalizations are a matter of utility. The preferential outcome of one instance of a generalized reaction over a set of other plausible reactions, is defined as chemoselectivity...
among functional groups. For example, sulfone
Sulfone
A sulfone is a chemical compound containing a sulfonyl functional group attached to two carbon atoms. The central hexavalent sulfur atom is double bonded to each of two oxygen atoms and has a single bond to each of two carbon atoms, usually in two separate hydrocarbon substituents.-IUPAC name and...
s and sulfoxide
Sulfoxide
A sulfoxide is a chemical compound containing a sulfinyl functional group attached to two carbon atoms. Sulfoxides can be considered as oxidized sulfides...
s can be reduced to the corresponding sulfide
Sulfide
A sulfide is an anion of sulfur in its lowest oxidation state of 2-. Sulfide is also a slightly archaic term for thioethers, a common type of organosulfur compound that are well known for their bad odors.- Properties :...
in the presence of a variety of carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
-containing functionalities (such as ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
s, ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s, amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
s, aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s, etc.). This is presumably due to the considerably slower reaction with carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
s as compared to sulfone
Sulfone
A sulfone is a chemical compound containing a sulfonyl functional group attached to two carbon atoms. The central hexavalent sulfur atom is double bonded to each of two oxygen atoms and has a single bond to each of two carbon atoms, usually in two separate hydrocarbon substituents.-IUPAC name and...
s and sulfoxide
Sulfoxide
A sulfoxide is a chemical compound containing a sulfinyl functional group attached to two carbon atoms. Sulfoxides can be considered as oxidized sulfides...
s. Furthermore, hydrodehalogenation of halogenated hydrocarbons to the corresponding hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
compound can be achieved using samarium(II) iodide. Also, it can be monitored by the color change that occurs as the dark blue color of SmI2 in THF discharges to a light yellow once the reaction has occurred. The picture shows the dark colour disappearing immediately upon contact with the Barbier reaction
Barbier reaction
The Barbier reaction is an organic reaction between an alkyl halide and a carbonyl group as an electrophilic substrate in the presence of magnesium, aluminium, zinc, indium, tin or its salts. The reaction product is a primary, secondary or tertiary alcohol...
mixture.
Work-up is with dilute hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
, and the samarium is removed as aqueous Sm3+.
Carbonyl compounds can also be coupled with simple alkenes to form five, six or eight membered rings.
Tosyl
Tosyl
A tosyl group is CH3C6H4SO2. This group is usually derived from the compound 4-toluenesulfonyl chloride, CH3C6H4SO2Cl, which forms esters and amides of toluenesulfonic or tosylic acid...
groups can be removed from N-tosylamides almost instantaneously, using SmI2 in conjunction with a base. The reaction is even effective for the synthesis of sensitive amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s such as aziridine
Aziridine
Aziridines are organic compounds containing the aziridine functional group, a three-membered heterocycle with one amine group and two methylene groups...
s:
In the Markó-Lam deoxygenation
Markó-Lam deoxygenation
The Markó–Lam deoxygenation is an organic chemistry reaction where the hydroxy functional group in an organic compound is replaced by a hydrogen atom to give an alkyl group. The Markó-Lam reaction is a variant of the Bouveault–Blanc reduction and an alternative to the classical Barton–McCombie...
, an alcohol could be almost instantaneously deoxygenated by reducing their toluate ester in presence of SmI2.
The applications of SmI2 have been reviewed.