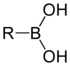
Boronic acid
Encyclopedia
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
substituted boric acid
Boric acid
Boric acid, also called hydrogen borate or boracic acid or orthoboric acid or acidum boricum, is a weak acid of boron often used as an antiseptic, insecticide, flame retardant, as a neutron absorber, and as a precursor of other chemical compounds. It exists in the form of colorless crystals or a...
containing a carbon–boron bond belonging to the larger class of organoborane
Organoborane
Organoborane or organoboron compounds are chemical compounds that are organic derivatives of BH3, for example trialkyl boranes. Organoboron chemistry or organoborane chemistry is the chemistry of these compounds...
s. Boronic acids act as Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
s. Their unique feature is that they are capable of forming reversible covalent complexes with sugar
Sugar
Sugar is a class of edible crystalline carbohydrates, mainly sucrose, lactose, and fructose, characterized by a sweet flavor.Sucrose in its refined form primarily comes from sugar cane and sugar beet...
s, amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s, hydroxamic acid
Hydroxamic acid
A hydroxamic acid is a class of chemical compounds sharing the same functional group in which an hydroxylamine is inserted into a carboxylic acid. Its general structure is R-CO-NH-OH, with an R as an organic residue, a CO as a carbonyl group, and a hydroxylamine as NH2-OH. They are used as metal...
s, etc. (molecules with vicinal
Vicinal (chemistry)
In chemistry vicinal stands for any two functional groups bonded to two adjacent carbon atoms. For example the molecule 2,3-dibromobutane carries two vicinal bromine atoms and 1,3-dibromobutane does not....
, (1,2) or occasionally (1,3) substituted Lewis base donors (alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
, amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
, carboxylate)). The pKa
PKA
PKA, pKa, or other similar variations may stand for:* pKa, the symbol for the acid dissociation constant at logarithmic scale* Protein kinase A, a class of cAMP-dependent enzymes* Pi Kappa Alpha, the North-American social fraternity...
of a boronic acid is ~9, but they can form tetrahedral
Tetrahedral molecular geometry
In a tetrahedral molecular geometry a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are cos−1 ≈ 109.5° when all four substituents are the same, as in CH4. This molecular geometry is common throughout the first...
boronate complexes with pKa ~7. They are occasionally used in the area of molecular recognition to bind to saccharides for fluorescent detection or selective transport of saccharides across membranes.
Boronic acids are used extensively in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
as chemical building blocks and intermediates predominantly in the Suzuki coupling. A key concept in its chemistry is transmetallation of its organic residue to a transition metal.
The compound bortezomib
Bortezomib
Bortezomib is the first therapeutic proteasome inhibitor to be tested in humans. It is approved in the U.S. for treating relapsed multiple myeloma and mantle cell lymphoma...
with a boronic acid group is a drug used in chemotherapy
Chemotherapy
Chemotherapy is the treatment of cancer with an antineoplastic drug or with a combination of such drugs into a standardized treatment regimen....
. The boron atom in this molecule is a key substructure because through it certain proteasome
Proteasome
Proteasomes are very large protein complexes inside all eukaryotes and archaea, and in some bacteria. In eukaryotes, they are located in the nucleus and the cytoplasm. The main function of the proteasome is to degrade unneeded or damaged proteins by proteolysis, a chemical reaction that breaks...
s are blocked that would otherwise degrade proteins.
Boronic acids
Many air-stable boronic acids are commercially available. They are characterised by high melting points. Since boronic acids easily lose water to form the cyclic trimeric anhydride, commercial material oftentimes contains substantial quantities of this anhydride. This does not affect reactivity.| Boronic acid | |R | |Molar mass Molar mass Molar mass, symbol M, is a physical property of a given substance , namely its mass per amount of substance. The base SI unit for mass is the kilogram and that for amount of substance is the mole. Thus, the derived unit for molar mass is kg/mol... |
CAS number | Melting point Melting point The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure... °C |
|
| Phenylboronic acid Phenylboronic acid Phenylboronic acid or benzeneboronic acid, abbreviated as PhB2 where Ph is the phenyl group C6H5-, is a boronic acid containing a phenyl substituent and two hydroxyl groups attached to boron. Phenylboronic acid is white powder and is commonly used in organic synthesis... |
Phenyl | 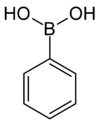 |
121.93 | 98-80-6 | 216–219 |
| 2-Thienylboronic acid | Thiophene Thiophene Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a flat five-membered ring, it is aromatic as indicated by its extensive substitution reactions. Related to thiophene are benzothiophene and dibenzothiophene, containing the thiophene ring fused with one and two benzene... |
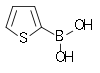 |
127.96 | 6165-68-0 | 138–140 |
| Methylboronic acid | Methyl | 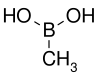 |
59.86 | 13061-96-6 | 91–94 |
| cis-Propenylboronic acid | propene | 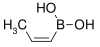 |
85.90 | 7547-96-8 | 65–70 |
| trans-Propenylboronic acid | propene | 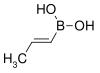 |
85.90 | 7547-97-9 | 123–127 |
| Representative boronic acids | |||||
Synthesis
Boronic acids can be obtained via several methods. The most common way is reaction of organometallic compounds based on lithium or magnesium (GrignardGrignard
Grignard can be a French last name, or refers to an organic chemical reaction.* Victor Grignard, a French organic chemist.* The Grignard Company a chemical manufacturer.* Grignard reaction, an organic chemical reaction developed by Victor Grignard....
s) with borate esters. For example phenylboronic acid
Phenylboronic acid
Phenylboronic acid or benzeneboronic acid, abbreviated as PhB2 where Ph is the phenyl group C6H5-, is a boronic acid containing a phenyl substituent and two hydroxyl groups attached to boron. Phenylboronic acid is white powder and is commonly used in organic synthesis...
is produced from phenylmagnesium bromide
Phenylmagnesium bromide
Phenylmagnesium bromide, with the simplified formula , is a magnesium-containing organometallic compound. It is so commonly used that it is commercially available as a solution in diethyl ether or tetrahydrofuran . Phenylmagnesium bromide is a Grignard reagent...
and trimethyl borate
Trimethyl borate
Trimethyl borate, or boron trimethoxide, has formula B3. It is a clear liquid. It melts at −34 °C and boils at 68-69 °C. It decomposes in contact with water...
followed by hydrolysis
- PhMgBr + B(OMe)3 → PhB(OMe)2 + MeOMgBr
- PhB(OMe)2 + H2O → PhB(OH)2 + MeOH
Another method is reaction of an arylsilane (RSiR3) with boron tribromide
Boron tribromide
Boron tribromide, BBr3, is a colorless, fuming liquid compound containing boron and bromine. It is usually made by heating boron trioxide with carbon in the presence of bromine: this generates free boron which reacts vigorously with the bromine...
(BBr3) in a transmetallation to RBBr2 followed by acidic hydrolysis.
A third method is by palladium catalysed reaction of aryl halides and triflates with diboronyl esters in a coupling reaction
Coupling reaction
A coupling reaction in organic chemistry is a catch-all term for a variety of reactions where two hydrocarbon fragments are coupled with the aid of a metal catalyst...
. An alternative to esters in this method is the use of diboronic acid or tetrahydroxydiboron ([B(OH2)]2).
Boronate esters
Boronate esters are esterEster
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
s formed between a boronic acid and an alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
.
| Compound | General formula | General structure |
|---|---|---|
| Boronic acid | RB(OH)2 |  |
| Boronate ester | RB(OR)2 | 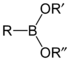 |
| Comparison between boronic acids and boronate esters | ||
The compounds can be obtained from borate esters by condensation with alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s and diol
Diol
A diol or glycol is a chemical compound containing two hydroxyl groups A geminal diol has two hydroxyl groups bonded to the same atom...
s. Phenylboronic acid can be selfcondensed to the cyclic trimer
Trimer (chemistry)
In chemistry, a trimer is a product derived from three identical precursors. Trimers are typically cyclic. Chemical compounds that often trimerise are aliphatic isocyanates and cyanic acids. Often, trimerization competes with polymerization....
called triphenyl anhydride or triphenylboroxin.
| Boronic ester | Diol | Structural formula | Molar mass Molar mass Molar mass, symbol M, is a physical property of a given substance , namely its mass per amount of substance. The base SI unit for mass is the kilogram and that for amount of substance is the mole. Thus, the derived unit for molar mass is kg/mol... |
CAS number | Boiling point Boiling point The boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid.... (°C) |
|---|---|---|---|---|---|
| Allylboronic acid pinacol ester | pinacol Pinacol Pinacol is a white solid organic compound.-Preparation:It may be produced by the pinacol coupling reaction from acetone:-Reactions:As a vicinal-diol, it can rearrange to pinacolone by the pinacol rearrangement, e.g... |
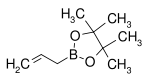 |
168.04 | 72824-04-5 | 50–53 (5 mmHg) |
| Phenyl boronic acid trimethylene glycol ester | trimethylene glycol | 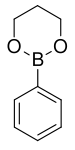 |
161.99 | 4406-77-3 | 106 (2 mm Hg) |
| Diisopropoxymethylborane | isopropanol | 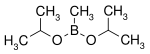 |
144.02 | 86595-27-9 | 105 -107 |
| Representative boronic esters | |||||
Compounds with 5-membered cyclic structures containing the C-O-B-O-C linkage are called dioxaborolanes and those with 6-membered rings dioxaborinanes.
Suzuki coupling reaction
Boronic acids are used in organic chemistryOrganic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
in the Suzuki reaction
Suzuki reaction
The Suzuki reaction is the organic reaction of an aryl- or vinyl-boronic acid with an aryl- or vinyl-halide catalyzed by a palladium complex. It is widely used to synthesize poly-olefins, styrenes, and substituted biphenyls, and has been extended to incorporate alkyl bromides...
. In this reaction the boron atom exchanges its aryl group with an alkoxy group from palladium.
Chan-Lam coupling
In the Chan-Lam coupling the alkyl, alkenyl or aryl boronic acid reacts with a N-H or O-H containing compound with Cu(II) such as copper(II) acetateCopper(II) acetate
Copper acetate, also referred to as cupric acetate, is the chemical compound with the formula Cu2 where OAc- is acetate . The hydrated derivative, which contains one molecule of water for each Cu atom, is available commercially. Anhydrous Cu2 is a dark green crystalline solid, whereas Cu22 is...
and oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
and a base such as pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
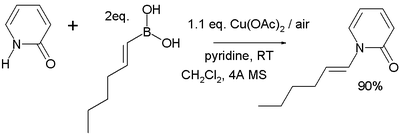
forming a new carbon–nitrogen bond or carbon–oxygen bond for example in this reaction of 2-pyridone
2-Pyridone
2-Pyridone is an organic compound with the formula . This colourless crystalline solid is used in peptide synthesis. It is well known to form hydrogen bonded structures somewhat related to the base-pairing mechanism found in RNA and DNA...
with trans-1-hexenylboronic acid:
The reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
sequence is deprotonation
Deprotonation
Deprotonation is the removal of a proton from a molecule, forming the conjugate base.The relative ability of a molecule to give up a proton is measured by its pKa value. A low pKa value indicates that the compound is acidic and will easily give up its proton to a base...
of the amine, coordination of the amine to the copper(II), transmetallation (transferring the alkyl boron group to copper and the copper acetate group to boron), oxidation of Cu(II) to Cu(III) by oxygen and finally reductive elimination of Cu(III) to Cu(I) with formation of the product. Direct reductive elimination of Cu(II) to Cu(0) also takes place but is very slow. In catalytic systems
Catalytic cycle
A catalytic cycle in chemistry is a term for a multistep reaction mechanism that involves a catalyst . The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, materials science, etc. Often such cycles show the conversion of a...
oxygen also regenerates the Cu(II) catalyst.
Liebeskind-Srogl coupling
In the Liebeskind-Srogl couplingLiebeskind-Srogl coupling
The Liebeskind–Srogl coupling reaction is an organic reaction forming a new carbon-carbon bond from a thioester and a boronic acid using a metal catalyst. This reaction was invented and named after Jiri Srogl from the Academy of Sciences, Czech Republic, and Lanny S. Liebeskind from Emory...
a thiol ester is coupled with a boronic acid to produce a ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
.
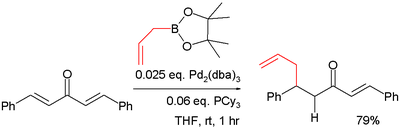
Conjugate addition
The boronic acid organic residue is a nucleophile in conjugate addition also in conjunction with a metal. In one study the pinacol ester of allylboronic acid is reacted with dibenzylidene acetone in a such a conjugate addition :- The catalyst system in this reaction is tris(dibenzylideneacetone)dipalladium(0)Tris(dibenzylideneacetone)dipalladium(0)Trisdipalladium or Pd23 is an organometallic complex based on palladium and dibenzylideneacetone used in organic chemistry. It was discovered in 1970.-Preparation and structure:...
/ tricyclohexylphosphineTricyclohexylphosphineTricyclohexylphosphine is the tertiary phosphine with the formula P3. Commonly used as a ligand in organometallic chemistry, it is often abbreviated to PCy3, where Cy stands for cyclohexyl...
.
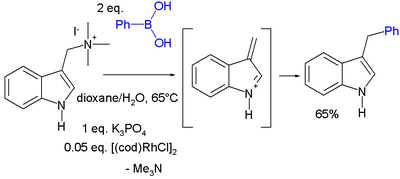
Another conjugate addition is that of gramine
Gramine
Gramine is a naturally occurring indole alkaloid present in several plant species. Gramine may play a defensive role in these plants, since it is toxic to many organisms.-Occurrence:...
with phenylboronic acid catalyzed by cyclooctadiene rhodium chloride dimer :
Oxidation
Boronic esters are oxidized to the corresponding alcoholAlcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s with base and hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
(for an example see: carbenoid
Carbenoid
In chemistry a carbenoid is a reactive intermediate that shares reaction characteristics with a carbene. In the Simmons-Smith reaction the carbenoid intermediate is a zinc / iodine complex that takes the form of...
)
Homologization
- In boronic ester homologization an alkyl group shiftsRearrangement reactionA rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
from boron in a boronate to carbon :
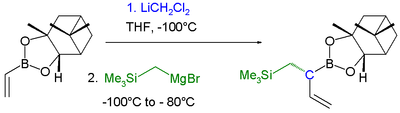
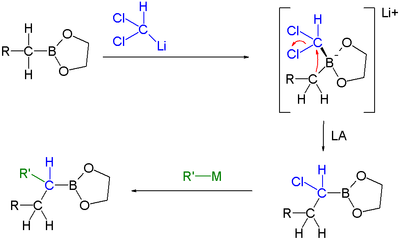 |
 |
|
| Boronic ester homologization mechanism | Homologization application | |
In this reaction dichloromethyllithium converts the boronic ester into a boronate. A lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
then induces a rearrangement of the alkyl group with displacement
Single displacement reaction
A single-displacement reaction, also called single-replacement reaction, is a type of oxidation-reduction chemical reaction when an element or ion moves out of one compound and into another. This is usually written as...This will occur if A is more reactive than B...
of the chlorine group. Finally an organometallic reagent such as a Grignard reagent displaces the second chlorine atom effectively leading to insertion of an RCH2 group into the C-B bond. Another reaction featuring a boronate alkyl migration is the Petasis reaction
Petasis reaction
The Petasis reaction is the chemical reaction of an amine, aldehyde, and vinyl- or aryl-boronic acid to form substituted amines.Reported in 1993 by N.A. Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine, the Petasis reaction can be described as...
.
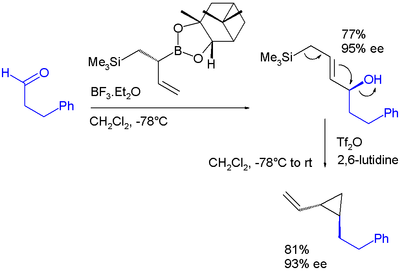
Electrophilic allyl shifts
Allyl boronic esters engage in electrophilic allyl shifts very much like silicon pendant in the Sakurai reactionSakurai reaction
The Sakurai reaction is the chemical reaction of carbon electrophiles with allylic silanes catalyzed by strong Lewis acids. It is named after the chemists Akira Hosomi and Hideki Sakurai.Lewis acid activation is essential for complete reaction...
. In one study a diallylation reagent combines both :
Hydrolysis
HydrolysisHydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
of boronic esters back to the boronic acid and the alcohol can be accomplished in certain systems with thionyl chloride
Thionyl chloride
Thionyl chloride is an inorganic compound with the formula SOCl2. It is a reactive chemical reagent used in chlorination reactions. It is a colorless, distillable liquid at room temperature and pressure that decomposes above 140 °C. Thionyl chloride is sometimes confused with sulfuryl...
and pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
.
Aryl Boronic acids or esters may be hydrolyzed to the corresponding phenols
Phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of a hydroxyl group bonded directly to an aromatic hydrocarbon group...
by reaction with hydroxylamine
Hydroxylamine
Hydroxylamine is an inorganic compound with the formula NH2OH. The pure material is a white, unstable crystalline, hygroscopic compound. However, hydroxylamine is almost always provided and used as an aqueous solution. It is used to prepare oximes, an important functional group. It is also an...
at room temperature.
C-H coupling reactions
The diboron compound bis(pinacolato)diboronBis(pinacolato)diboron
Bisdiboron is a boron containing two pinacolato ligands. This compound may be prepared by reacting tetrakisdiboron with pinacol in acidic conditions. This compound is a commercially-available starting material useful for making Pinacol boronic esters for organic synthesis....
reacts with aromatic heterocycles or simple arene
Aromatic hydrocarbon
An aromatic hydrocarbon or arene is a hydrocarbon with alternating double and single bonds between carbon atoms. The term 'aromatic' was assigned before the physical mechanism determining aromaticity was discovered, and was derived from the fact that many of the compounds have a sweet scent...
s to an arylboronate ester with iridium
Iridium
Iridium is the chemical element with atomic number 77, and is represented by the symbol Ir. A very hard, brittle, silvery-white transition metal of the platinum family, iridium is the second-densest element and is the most corrosion-resistant metal, even at temperatures as high as 2000 °C...
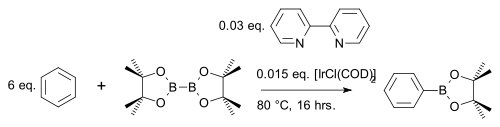
catalyst [IrCl(COD)]2 (a modification of Crabtree's catalyst
Crabtree's catalyst
Crabtree's catalyst is the name given to a complex of iridium with 1,5-cyclooctadiene, tris-cyclohexylphosphine, and pyridine. It is a homogeneous catalyst for hydrogenation reactions, developed by Robert H. Crabtree, a professor at Yale University...
) and base 4,4′-di-tert-butyl-2,2′-bipyridine in a C-H coupling reaction
Coupling reaction
A coupling reaction in organic chemistry is a catch-all term for a variety of reactions where two hydrocarbon fragments are coupled with the aid of a metal catalyst...
for example with benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
:
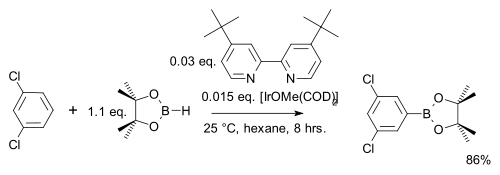
In one modification the arene reacts 1 on 1 (instead of a large excess) with cheaper pinacolborane
Unlike in ordinary electrophilic aromatic substitution
Electrophilic aromatic substitution
Electrophilic aromatic substitution EAS is an organic reaction in which an atom, usually hydrogen, appended to an aromatic system is replaced by an electrophile...
(EAS) where electronic effect
Electronic effect
An electronic effect influences the structure, reactivity, or properties of molecule but is neither a traditional bond nor a steric effect. In organic chemistry, the term stereoelectronic effect is also used to emphasize the relation between the electronic structure and the geometry of a...
s dominate, the regioselectivity
Regioselectivity
In chemistry, regioselectivity is the preference of one direction of chemical bond making or breaking over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base will abstract from an organic molecule, or where...
in this reaction type is solely determined by the steric bulk of the iridium complex. This is exploited in a meta-bromination of m-xylene
M-Xylene
m-Xylene is an aromatic hydrocarbon, based on benzene with two methyl substituents.It is an isomer of o-xylene and p-xylene. The m stands for meta, meaning the two methyl substituents are at locants 1 and 3 on the aromatic ring....
which by standard AES would give the ortho product:
Saccharide recognition

Dynamic covalent chemistry
In supramolecular chemistry, dynamic covalent chemistry is a strategy that aims at synthesizing large complex molecules. In it a reversible reaction is under thermodynamic reaction control and a specific reaction product out of many is captured...
strategy lies in the ability of boronic acids to overcome the challenge of binding neutral species in aqueous media. If arranged correctly, the introduction of a tertiary amine within these supramolecular
Supramolecular chemistry
Supramolecular chemistry refers to the area of chemistry beyond the molecules and focuses on the chemical systems made up of a discrete number of assembled molecular subunits or components...
systems will permit binding to occur at physiological pH and allow signalling mechanisms such as photoinduced electron transfer
Photoinduced electron transfer
Photoinduced electron transfer is an electron transfer which occurs when certain photoactive materials interact with light. - Breadth :Such materials include semiconductors that can be photoactivated like many solar cells, biological systems such as those used in photosynthesis, and small...
mediated fluorescence
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. It is a form of luminescence. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation...
emission to report the binding event.
Potential applications for this research include systems to monitor diabetic
Diabetes mellitus
Diabetes mellitus, often simply referred to as diabetes, is a group of metabolic diseases in which a person has high blood sugar, either because the body does not produce enough insulin, or because cells do not respond to the insulin that is produced...
blood glucose
Blood glucose monitoring
Blood glucose monitoring is a way of testing the concentration of glucose in the blood . Particularly important in the care of diabetes mellitus, a blood glucose test is performed by piercing the skin to draw blood, then applying the blood to a chemically active disposable 'test-strip'...
levels. As the sensors employ an optical response, monitoring could be achieved using minimally invasive methods, one such example is the investigation of a contact lens doped with boronic acid based sensors to monitor glucose levels within ocular fluid.
Borinic acids and esters
Borinic acids and borinate esters have the general structure R2BOR.| compound | general formula | general structure |
| borinic acid | R2BOH |  |
| borinate ester | R2BOR | 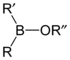 |
Borate salts
Borate salts are ate complexAte complex
An ate complex in chemistry is a salt formed by reaction of a Lewis acid with a base whereby the central atom increases its valence . Often in chemical nomenclature the phrase ate is suffixed to the element in question. For example, the ate complex of a boron compound is called a borate...
es and have the general structure R4B-M+ for example potassium tetraphenylborate
Potassium tetraphenylborate
Potassium tetraphenylborate is a water-insoluble salt of potassium . It is, however, soluble in organic solvents....
(IUPAC name: potassium tetraphenylboranuide).
See also
- BoronBoronBoron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the...
bonded to three oxygen atoms: boric acidBoric acidBoric acid, also called hydrogen borate or boracic acid or orthoboric acid or acidum boricum, is a weak acid of boron often used as an antiseptic, insecticide, flame retardant, as a neutron absorber, and as a precursor of other chemical compounds. It exists in the form of colorless crystals or a...
and borateBorateBorates are chemical compounds which contain oxoanions of boron in oxidation state +3. The simplest borate ion, BO33−, has a trigonal planar structure. Other borates are made up of trigonal BO3 or tetrahedral BO4 structural units, sharing oxygen atoms...
s - Suzuki reactionSuzuki reactionThe Suzuki reaction is the organic reaction of an aryl- or vinyl-boronic acid with an aryl- or vinyl-halide catalyzed by a palladium complex. It is widely used to synthesize poly-olefins, styrenes, and substituted biphenyls, and has been extended to incorporate alkyl bromides...
- Supramolecular chemistrySupramolecular chemistrySupramolecular chemistry refers to the area of chemistry beyond the molecules and focuses on the chemical systems made up of a discrete number of assembled molecular subunits or components...
- Dynamic covalent chemistryDynamic covalent chemistryIn supramolecular chemistry, dynamic covalent chemistry is a strategy that aims at synthesizing large complex molecules. In it a reversible reaction is under thermodynamic reaction control and a specific reaction product out of many is captured...
- Blood glucose monitoringBlood glucose monitoringBlood glucose monitoring is a way of testing the concentration of glucose in the blood . Particularly important in the care of diabetes mellitus, a blood glucose test is performed by piercing the skin to draw blood, then applying the blood to a chemically active disposable 'test-strip'...