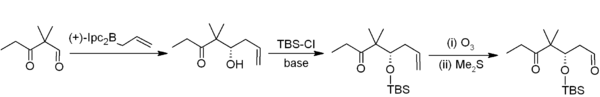Organoborane
Encyclopedia
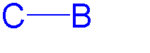
Properties
The C-B bond has low polarity (the difference in electronegativityElectronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
2.55 for carbon and 2.04 for boron) and therefore alkyl boron compounds are in general stable though easily oxidized. Vinyl
Vinyl
A vinyl compound is any organic compound that contains a vinyl group ,which are derivatives of ethene, CH2=CH2, with one hydrogen atom replaced with some other group...
groups and aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
groups donate electrons and make boron less electrophilic and the C-B bond gains some double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
character. Like the parent borane, diborane
Diborane
Diborane is the chemical compound consisting of boron and hydrogen with the formula B2H6. It is a colorless gas at room temperature with a repulsively sweet odor. Diborane mixes well with air, easily forming explosive mixtures. Diborane will ignite spontaneously in moist air at room temperature...
, organoboranes are classified in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
as strong electrophile
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
s because boron is unable to gain a full octet
Octet rule
The octet rule is a chemical rule of thumb that states that atoms of low The octet rule is a chemical rule of thumb that states that atoms of low The octet rule is a chemical rule of thumb that states that atoms of low (The octet rule is a chemical rule of thumb that states that atoms of low (...
of electrons. Unlike diborane however, organoboranes do not form dimers.
Other boranes of interest are carborane
Carborane
A carborane is a cluster composed of boron and carbon atoms. Like many of the related boranes, these clusters are polyhedra and are similarly classified as closo-, nido-, arachno-, hypho-, etc...
s, cluster compounds of carbon and boron and borabenzene
Borabenzene
A borabenzene is a heteroaromatic compound that has a boron atom instead of the carbon atom of a benzene molecule. A free borabenzene, which has no donor ligand on the boron atom, has not yet been isolated despite its simple structure and the chemical robustness of boron-carbon bonds...
, the boron equivalent of benzene.
Organoboranes with carbon replaced by oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
are borinic esters R2BOR, boronic esters RB(OR)2 and borate
Borate
Borates are chemical compounds which contain oxoanions of boron in oxidation state +3. The simplest borate ion, BO33−, has a trigonal planar structure. Other borates are made up of trigonal BO3 or tetrahedral BO4 structural units, sharing oxygen atoms...
s B(OR)3 such as trimethylborate. In organometallic chemistry
Organometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
compounds with metal to boron bonds are called boryls (M–BR2) or borylenes (M–B(R)–M).
From Grignards
Simple organoboranes such as triethylboraneTriethylborane
Triethylborane , also called triethylborine and triethylboron, is an organoborane , a near-colorless to yellowish transparent liquid with pungent ether-like odor. Its chemical formula can be written as C6H15B, or 3B, or 3B, or Et3B.Triethylborane is strongly pyrophoric, igniting spontaneously in...
or tris(pentafluorophenyl)boron
Tris(pentafluorophenyl)boron
Trisboron is the chemical compound 3B. The molecule consists of three pentafluorophenyl groups attached in a "paddle-wheel" manner to a central boron atom; the BC3 core is planar. It has been described as the “ideal Lewis acid” because of its versatility and the relative inertness of the B-C bonds...
can be prepared from trifluoroborane (as the ether
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
complex) and the ethyl or pentafluorophenyl Grignard reagent.
From alkenes
Boranes react rapidly to alkeneAlkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s in a process called hydroboration. This concept was discovered by Dr. Herbert Charles Brown at Purdue University
Purdue University
Purdue University, located in West Lafayette, Indiana, U.S., is the flagship university of the six-campus Purdue University system. Purdue was founded on May 6, 1869, as a land-grant university when the Indiana General Assembly, taking advantage of the Morrill Act, accepted a donation of land and...
, work for which he eventually received the Nobel Prize
Nobel Prize
The Nobel Prizes are annual international awards bestowed by Scandinavian committees in recognition of cultural and scientific advances. The will of the Swedish chemist Alfred Nobel, the inventor of dynamite, established the prizes in 1895...
(jointly with Georg Wittig
Georg Wittig
Georg Wittig was a German chemist who reported a method for synthesis of alkenes from aldehydes and ketones using compounds called phosphonium ylides in the Wittig reaction. He shared the Nobel Prize in Chemistry with Herbert C...
for his discovery of the Wittig reaction
Wittig reaction
The Wittig reaction is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide to give an alkene and triphenylphosphine oxide....
). Although diborane as a pure compound is a dimer, BH3 forms 1:1 complexes with basic solvents, for instance THF
ThF
Follicular B helper T cells , are antigen-experienced CD4+ T cells found in the B cell follicles of secondary lymphoid organs such as lymph nodes, spleens and Peyer's patches, and are identified by their constitutive expression of the B cell follicle homing receptor CXCR5...
. In an ordinary electrophilic addition
Electrophilic addition
In organic chemistry, an electrophilic addition reaction is an addition reaction where, in a chemical compound, a π bond is broken and two new σ bonds are formed...
reaction of HX (X = Cl, Br, I, etc.) the Markovnikov's rule
Markovnikov's rule
In organic chemistry, Markovnikov's rule or Markownikoff's rule is an observation based on Zaitsev's rule. It was formulated by the Russian chemist Vladimir Vasilevich Markovnikov in 1870....
, which states that the lest electronegative atom, usually hydrogen adds to the least substituted carbon of the double bond, this determines regioselectivity
Regioselectivity
In chemistry, regioselectivity is the preference of one direction of chemical bond making or breaking over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base will abstract from an organic molecule, or where...
. With boranes the mode of action is the same, the hydrogen adds to the most-substituted carbon because boron is least electronegative than hydrogen. The reason is that boron is less electronegative than hydrogen. When a positive charge develops in the alkene on the most substituted carbon atom, that is where the partially negatively charged hydrogen atom adds, leaving the least substituted carbon atom for the boron atom. The so called anti-Markovnikov addition because when the boron is replaced with a hydroxyl group the overall reaction is addition of water over the double bond in what appears to be an anti.Makovnikov addition.
This is most pronounced when the boron compound has very bulky substituents. One organoboron reagent that is often employed in synthesis is 9-borabicyclo[3.3.1]nonane or 9-BBN
9-BBN
9-Borabicyclo[3.3.1]nonane or 9-BBN is an organoborane compound. This colourless solid is used in organic chemistry as a hydroboration reagent. The compound exists as a hydride-bridged dimer, which easily cleaves in the presence of reducible substrates...
which is generated from the reaction of cyclooctadiene
Cyclooctadiene
A cyclooctadiene is a cyclic diene with the formula C8H12. Focusing only on cis derivatives, four isomers are possible: 1,2, which is an allene, 1,3-, 1,4-, and 1,5-. Commonly encountered isomers are 1,3-cyclooctadiene and 1,5-cyclooctadiene, which is used as a ligand for transition...
and diborane
Diborane
Diborane is the chemical compound consisting of boron and hydrogen with the formula B2H6. It is a colorless gas at room temperature with a repulsively sweet odor. Diborane mixes well with air, easily forming explosive mixtures. Diborane will ignite spontaneously in moist air at room temperature...
. Hydroborations take place stereospecifically
Stereospecificity
In chemistry, stereospecificity is the property of a reaction mechanism that leads to different stereoisomeric reaction products from different stereoisomeric reactants, or which operates on only one of the stereoisomers."Overlap Control of Carbanionoid Reactions. I. Stereoselectivity in Alkaline...
in a syn mode, that is on the same face of the alkene. In this concerted reaction
Concerted reaction
In chemistry, a concerted reaction is a chemical reaction in which all bond breaking and bond making occurs in a single step. Reactive intermediates or other unstable high energy intermediates are not involved. Concerted reaction rates tend not to depend on solvent polarity ruling out large buildup...
the transition state
Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
is represented as a square with the corners occupied by carbon, carbon, hydrogen and boron with maximum overlap between the two olefin p-orbitals and the empty boron orbital.
Hydroboration-oxidation
In organic synthesis the hydroboration reaction is taken further to generate other functional groups in the place of the boron group. The Hydroboration-oxidation reactionHydroboration-oxidation reaction
In organic chemistry, the hydroboration–oxidation reaction is a two-step organic reaction that converts an alkene into a neutral alcohol by the net addition of water across the double bond. The hydrogen and hydroxyl group are added in a syn addition leading to cis stereochemistry...
offers a route to alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s by oxidation of the borane with hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
or to the carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
group with the stronger oxidizing agent
Oxidizing agent
An oxidizing agent can be defined as a substance that removes electrons from another reactant in a redox chemical reaction...
chromium oxide
Chromium oxide
Chromium oxide may refer to:* Chromium oxide, CrO* Chromium oxide, Cr2O3* Chromium dioxide , CrO2* Chromium trioxide , CrO3* Chromium oxide peroxide, CrO5* Mixed valence species, such as Cr8O21...
.
Rearrangements
A second group of reactions that organoboron compounds are involved in create new carbon carbon bonds. Carbon monoxideCarbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
is found to react very easily with a trialkylborane. What follows is a 1,2-rearrangement
1,2-rearrangement
A 1,2-rearrangement or 1,2-migration or 1,2-shift or Whitmore 1,2-shift is an organic reaction where a substituent moves from one atom to another atom in a chemical compound. In a 1,2 shift the movement involves two adjacent atoms but moves over larger distances are possible...
when an alkyl substituent on the anionic boron migrates to the adjacent electrophilic carbon of the carbonyl group. The carbonyl group can then be reduced to a hydroxyl group .
Allylboration
Asymmetric allylboration demonstrates another useful application of organoboranes in carbon–carbon bond formation. In this example from Nicolaou's synthesis of the epothiloneEpothilone
The epothilones are a new class of cancer drugs. Like taxanes, they prevent cancer cells from dividing by interfering with tubulin, but in early trials epithilones have better efficacy and milder adverse effects than taxanes....
s, asymmetric allylboration (using an allylborane derived from chiral alpha-pinene
Alpha-Pinene
α-Pinene is an organic compound of the terpene class, one of two isomers of pinene. It is an alkene and it contains a reactive four-membered ring. It is found in the oils of many species of many coniferous trees, notably the pine. It is also found in the essential oil of rosemary...
) is used in conjunction with TBS protection
Silyl ether
Silyl ethers are a group of chemical compounds which contain a silicon atom covalently bonded to an alkoxy group. The general structure is R1R2R3Si−O−R4 where R4 is an alkyl group or an aryl group. Silyl ethers are usually used as protecting groups for alcohols in organic synthesis...
and ozonolysis
Ozonolysis
Ozonolysis is the cleavage of an alkene or alkyne with ozone to form organic compounds in which the multiple carbon–carbon bond has been replaced by a double bond to oxygen...
. Overall, this provides a two-carbon homologation sequence that delivers the required acetogenin sequence.
Transmetallation
Organoboron compounds also lend themselves to transmetalationTransmetalation
Transmetalation is a general chemical reaction type in organometallic chemistry describing the exchange of ligands between two metal centers....
reactions with organopalladium
Organopalladium
Organopalladium chemistry is a branch of organometallic chemistry that deals with organic palladium compounds and their reactions. Palladium is often used as a catalyst in the reduction of alkenes and alkynes with hydrogen. This process involves the formation of a palladium-carbon covalent bond...
compounds. This reaction type is exemplified in the Suzuki reaction
Suzuki reaction
The Suzuki reaction is the organic reaction of an aryl- or vinyl-boronic acid with an aryl- or vinyl-halide catalyzed by a palladium complex. It is widely used to synthesize poly-olefins, styrenes, and substituted biphenyls, and has been extended to incorporate alkyl bromides...
.
As reducing agent
Borane hydrides such as 9-BBN9-BBN
9-Borabicyclo[3.3.1]nonane or 9-BBN is an organoborane compound. This colourless solid is used in organic chemistry as a hydroboration reagent. The compound exists as a hydride-bridged dimer, which easily cleaves in the presence of reducible substrates...
and L-selectride
L-selectride
L-selectride is an organoborane. It is used in organic chemistry as a reducing agent, for example in the reduction of a ketone, as part of Overman's synthesis of strychnine....
(lithium tri-sec-butylborohydride) are reducing agent
Reducing agent
A reducing agent is the element or compound in a reduction-oxidation reaction that donates an electron to another species; however, since the reducer loses an electron we say it is "oxidized"...
s. An example of an asymmetric catalyst for carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
reductions is the CBS catalyst
CBS catalyst
The CBS catalyst or Corey-Bakshi-Shibata catalyst is an asymmetric catalyst derived from proline. It finds many uses in organic reactions such as the CBS reduction, Diels-Alder reactions and [3+2] cycloadditions. Proline, a naturally occurring chiral compound, is readily and cheaply available...
. This catalyst is also based on boron, the purpose of which is coordination to the carbonyl oxygen atom.
Borates
Trialkylboranes, BR3, can be oxidized to the corresponding borateBorate
Borates are chemical compounds which contain oxoanions of boron in oxidation state +3. The simplest borate ion, BO33−, has a trigonal planar structure. Other borates are made up of trigonal BO3 or tetrahedral BO4 structural units, sharing oxygen atoms...
s, B(OR)3. One method for the determination of the amount of C-B bonds in a compound is by oxidation of R3B with trimethylamine oxide (Me3NO) to B(OR)3. The trimethylamine
Trimethylamine
Trimethylamine is an organic compound with the formula N3. This colorless, hygroscopic, and flammable tertiary amine has a strong "fishy" odor in low concentrations and an ammonia-like odor at higher concentrations...
(Me3N) formed can then be titrated
Titration
Titration, also known as titrimetry, is a common laboratory method of quantitative chemical analysis that is used to determine the unknown concentration of an identified analyte. Because volume measurements play a key role in titration, it is also known as volumetric analysis. A reagent, called the...
.
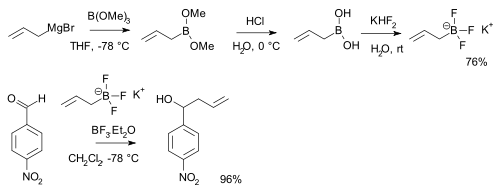
Boronic acid
Boronic acid
A boronic acid is an alkyl or aryl substituted boric acid containing a carbon–boron bond belonging to the larger class of organoboranes. Boronic acids act as Lewis acids. Their unique feature is that they are capable of forming reversible covalent complexes with sugars, amino acids, hydroxamic...
s RB(OH)2 react with potassium bifluoride
Potassium bifluoride
Potassium bifluoride is the inorganic compound with the formula KHF2. This colourless salt consists of the potassium cation and the bifluoride anion. The salt is used in etchant for glass...
K[HF2] to form trifluoroborate salts
Organotrifluoroborate
Organotrifluoroborates are organoboron compounds that contain an anion with the general formula [RBF3]−. They can be thought of as protected boronic acids, or as adducts of carbanions and boron trifluoride. Organotrifluoroborates are tolerant of air and moisture and are easy to handle and purify...
K[RBF3] which are precursors to nucleophilic alkyl and aryl boron difluorides, ArBF2. The salts are more stable than the boronic acids themselves and used for instance in alkylation of certain aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s:
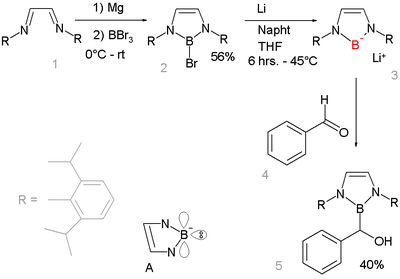
Boryllithium
Nucleophilic anionic boryl compounds have long been elusive but a 2006 study described a boryllithium compound which reacts as a nucleophileNucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
:
This is remarkable because in other period 2 element
Period 2 element
A period 2 element is one of the chemical elements in the second row of the periodic table. The periodic table is laid out in rows to illustrate recurring trends in the chemical behavior of the elements as their atomic number increases; a new row is started when chemical behavior begins to...
s lithium salts are common e.g. lithium fluoride
Lithium fluoride
Lithium fluoride is an inorganic compound with the formula LiF. It is the lithium salt of hydrofluoric acid. This white solid is a simple ionic compound. Its structure is analogous to that of sodium chloride, but it is much less soluble in water. It is mainly used as a component of molten...
, lithium hydroxide
Lithium hydroxide
Lithium hydroxide is an inorganic compound with the formula LiOH. It is a white hygroscopic crystalline material. It is soluble in water and slightly soluble in ethanol...
lithium amide
Lithium amide
Lithium amide is an inorganic compound with the chemical formula Li+NH2-, i.e. it is composed of a lithium cation, and the conjugate base of ammonia. It is a white solid with a tetragonal crystal structure.-Lithium amides:...
and methyllithium. Reaction of base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
with a borohydride R2BH does not result in deprotonation
Deprotonation
Deprotonation is the removal of a proton from a molecule, forming the conjugate base.The relative ability of a molecule to give up a proton is measured by its pKa value. A low pKa value indicates that the compound is acidic and will easily give up its proton to a base...
to the boryl anion R2B- but to formation of the boryl anion R2B-H(base)+ because only this reaction path gives a complete octet
Octet rule
The octet rule is a chemical rule of thumb that states that atoms of low The octet rule is a chemical rule of thumb that states that atoms of low The octet rule is a chemical rule of thumb that states that atoms of low (The octet rule is a chemical rule of thumb that states that atoms of low (...
. Instead the boryl compound is prepared by reductive heterolysis
Heterolysis
In chemistry, heterolysis or heterolytic fission is chemical bond cleavage of a neutral molecule generating a cation and an anion. In this process the two electrons that make up the bond are assigned to the same fragment...
of a boron-bromide bond by lithium
Lithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
metal. The new boryl lithium compound is very similar to and isoelectronic with N-heterocyclic carbenes. It is designed to benefit from aromatic stabilization
Aromaticity
In organic chemistry, Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August...
(6-electron system counting the nitrogen lone pairs and an empty boron p-orbital, see structure A) and from kinetic stabilization from the bulky 2,6-diisopropylphenyl groups. X-ray diffraction confirms sp2 hybridization at boron and its nucleophilic addition
Nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile....
reaction with benzaldehyde
Benzaldehyde
Benzaldehyde is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful. This colorless liquid has a characteristic pleasant almond-like odor...
gives further proof of the proposed structure.
Alkylideneboranes
Alkylideneboranes of the type RB=CRR with a boron – carbon double bondDouble bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
are rarely encountered. An example is borabenzene
Borabenzene
A borabenzene is a heteroaromatic compound that has a boron atom instead of the carbon atom of a benzene molecule. A free borabenzene, which has no donor ligand on the boron atom, has not yet been isolated despite its simple structure and the chemical robustness of boron-carbon bonds...
. The parent compound is HB=CH2 which can be detected at low temperatures. A fairly stable derivative is CH3B=C(SiMe3)2 but is prone to cyclodimerisation .
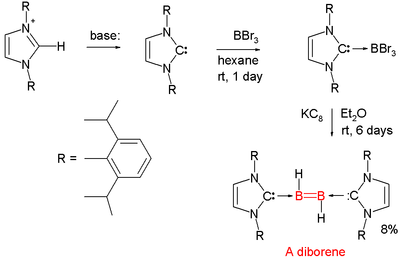
Diborenes
Chemical compounds with boron to boron double bondDouble bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
s are rare. In 2007 the first neutral diborene (RHB=BHR) was presented . Each boron atom has a proton attached to it and each boron atom is coordinated to a so-called NHC carbene.
Boroles
The cyclic compound boroleBorole
Borole is a - for the moment - theoretical heterocyclic organic compound, a five-membered ring with the formula C4H4BH. It is classified as a metallole. It can be viewed as an structural analog of pyrrole, with boron replacing the nitrogen atom of pyrrole. The unsubstituted compound has not been...
, a structural analog of pyrrole
Pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4H4NH. It is a colourless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3...
, has not been isolated, but substituted derivatives known as boroles are known.
Other uses
TEB – TriethylboraneTriethylborane
Triethylborane , also called triethylborine and triethylboron, is an organoborane , a near-colorless to yellowish transparent liquid with pungent ether-like odor. Its chemical formula can be written as C6H15B, or 3B, or 3B, or Et3B.Triethylborane is strongly pyrophoric, igniting spontaneously in...
was used to ignite the JP-7 fuel of the Pratt / Whitney J-58 ramjet engines powering the Lockheed SR-71 Blackbird.
See also
- Compounds of carbon with other elements in the periodic table: