
Dynamic covalent chemistry
Encyclopedia
In supramolecular chemistry
, dynamic covalent chemistry is a strategy that aims at synthesizing
large complex molecule
s. In it a reversible reaction
is under thermodynamic reaction control
and a specific reaction product out of many is captured. Because all the components in the reaction mixture are able to equilibrate quickly, (according to its advocates) some degree of error checking and proof reading is enabled. The concept of dynamic covalent chemistry was demonstrated in the development of specific molecular Borromean rings
.
The underlying idea is that rapid equilibration allows the coexistence of a huge variety of different species among which one can select molecules with desired chemical, pharmaceutical and biological properties. For instance, the addition of a proper template will shift the equilibrium toward the component that forms the complex of higher stability (thermodynamic template effect). After the new equilibrium
is established, the researcher
modifies the reaction conditions so as to stop equilibration. The optimal binder for the template is then extracted from the reactional mixture by the usual laboratory
procedures.
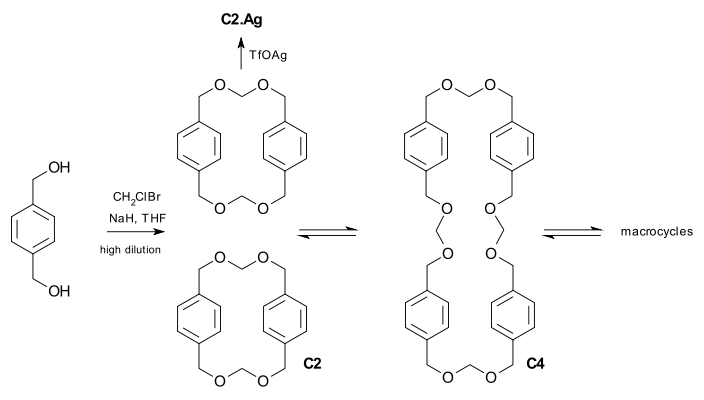
The concept is demonstrated in a minor but illustrative example involving polyacetal
macrocycle
s. The cyclophane
C2 can be prepared by the irreversible highly diluted reaction of a diol with chlorobromomethane in the presence of sodium hydride
. The dimer however is part of series of equilibria between polyacetal macrocycles of different size brought about by acid catalyzed
(triflic acid) transacetalization. Regardless of the starting material, C2, C4 or a high molar mass
product, the equilibrium will eventually produce an identical product distribution. In this system it is also possible to amplify the presence of C2 in the mixture when the catalyst is silver triflate because the silver ion fits ideally and irreversibly in its cavity
.
Supramolecular chemistry
Supramolecular chemistry refers to the area of chemistry beyond the molecules and focuses on the chemical systems made up of a discrete number of assembled molecular subunits or components...
, dynamic covalent chemistry is a strategy that aims at synthesizing
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
large complex molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s. In it a reversible reaction
Reversible reaction
A reversible reaction is a chemical reaction that results in an equilibrium mixture of reactants and products. For a reaction involving two reactants and two products this can be expressed symbolically as...
is under thermodynamic reaction control
Thermodynamic reaction control
Thermodynamic reaction control or kinetic reaction control in a chemical reaction can decide the composition in a reaction product mixture when competing pathways lead to different products and the reaction conditions influence the selectivity...
and a specific reaction product out of many is captured. Because all the components in the reaction mixture are able to equilibrate quickly, (according to its advocates) some degree of error checking and proof reading is enabled. The concept of dynamic covalent chemistry was demonstrated in the development of specific molecular Borromean rings
Molecular Borromean rings
Molecular Borromean rings are an example of a mechanically-interlocked molecular architecture in which three macrocycles are interlocked in such a way that breaking any macrocycle allows the others to disassociate. They are the smallest examples of Borromean rings. The synthesis of molecular...
.
The underlying idea is that rapid equilibration allows the coexistence of a huge variety of different species among which one can select molecules with desired chemical, pharmaceutical and biological properties. For instance, the addition of a proper template will shift the equilibrium toward the component that forms the complex of higher stability (thermodynamic template effect). After the new equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
is established, the researcher
Researcher
A researcher is somebody who performs research, the search for knowledge or in general any systematic investigation to establish facts. Researchers can work in academic, industrial, government, or private institutions.-Examples of research institutions:...
modifies the reaction conditions so as to stop equilibration. The optimal binder for the template is then extracted from the reactional mixture by the usual laboratory
Laboratory
A laboratory is a facility that provides controlled conditions in which scientific research, experiments, and measurement may be performed. The title of laboratory is also used for certain other facilities where the processes or equipment used are similar to those in scientific laboratories...
procedures.
The concept is demonstrated in a minor but illustrative example involving polyacetal
Polyoxymethylene
Polyoxymethylene , also known as acetal, polyacetal, and polyformaldehyde, is an engineering thermoplastic used in precision parts that require high stiffness, low friction and excellent dimensional stability....
macrocycle
Macrocycle
A macrocycle is, as defined by IUPAC, "a cyclic macromolecule or a macromolecular cyclic portion of a molecule." In the chemical literature, organic chemists may consider any molecule containing a ring of nine or more atoms to be a macrocycle...
s. The cyclophane
Cyclophane
A cyclophane is a hydrocarbon consisting of an aromatic unit and an aliphatic chain that forms a bridge between two non-adjacent positions of the aromatic ring. More complex derivatives with multiple aromatic units and bridges forming cagelike structures are also known...
C2 can be prepared by the irreversible highly diluted reaction of a diol with chlorobromomethane in the presence of sodium hydride
Sodium hydride
Sodium hydride is the chemical compound with the empirical formula NaH. It is primarily used as a strong base in organic synthesis. NaH is representative of the saline hydrides, meaning it is a salt-like hydride, composed of Na+ and H− ions, in contrast to the more molecular hydrides such as...
. The dimer however is part of series of equilibria between polyacetal macrocycles of different size brought about by acid catalyzed
Acid catalysis
In acid catalysis and base catalysis a chemical reaction is catalyzed by an acid or a base. The acid is often the proton and the base is often a hydroxyl ion. Typical reactions catalyzed by proton transfer are esterfications and aldol reactions. In these reactions the conjugate acid of the carbonyl...
(triflic acid) transacetalization. Regardless of the starting material, C2, C4 or a high molar mass
Molar mass
Molar mass, symbol M, is a physical property of a given substance , namely its mass per amount of substance. The base SI unit for mass is the kilogram and that for amount of substance is the mole. Thus, the derived unit for molar mass is kg/mol...
product, the equilibrium will eventually produce an identical product distribution. In this system it is also possible to amplify the presence of C2 in the mixture when the catalyst is silver triflate because the silver ion fits ideally and irreversibly in its cavity
Cavitand
A cavitand is a container shaped molecule. The cavity of the cavitand allows it to engage in host-guest chemistry with guest molecules of a complementary shape and size. Examples include cyclodextrins, calixarenes, pillararenes and cucurbiturils....
.

See also
- Organic chemistryOrganic chemistryOrganic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
- Supramolecular chemistrySupramolecular chemistrySupramolecular chemistry refers to the area of chemistry beyond the molecules and focuses on the chemical systems made up of a discrete number of assembled molecular subunits or components...
- Template effect
- Boronic acids in supramolecular chemistry: Saccharide recognition

