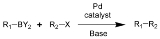
Suzuki reaction
Encyclopedia
The Suzuki reaction is the organic reaction
of an aryl
- or vinyl
-boronic acid
with an aryl
- or vinyl
-halide
catalyzed by a palladium(0) complex
. It is widely used to synthesize
poly-olefins, styrenes, and substituted biphenyl
s, and has been extended to incorporate alkyl bromides. Several reviews have been published.
 The reaction also works with pseudohalides, such as triflate
The reaction also works with pseudohalides, such as triflate
s (OTf), instead of halides. Boronic esters and organotrifluoroborate salts
may be used instead of boronic acids.
First published in 1979 by Akira Suzuki
, the Suzuki reaction couples
boronic acids (containing an organic part) to halides. The reaction relies on a palladium
catalyst such as tetrakis(triphenylphosphine)palladium(0)
to effect part of the transformation. The palladium catalyst (more strictly a pre-catalyst) is 4-coordinate, and usually involves phosphine
supporting groups.
The 2010 Nobel Prize in Chemistry
was awarded to Suzuki for his discovery and development of this reaction. In many publications this reaction also goes by the name Suzuki-Miyaura reaction. It is also often referred to as "Suzuki Coupling".
of the Suzuki reaction is best viewed from the perspective of the palladium catalyst. The first step is the oxidative addition
of palladium to the halide
2 to form the organopalladium
species 3. Reaction with base gives intermediate 4, which via transmetalation
with the boron-ate complex 6 forms the organopalladium
species 8. Reductive elimination of the desired product 9 restores the original palladium catalyst 1.
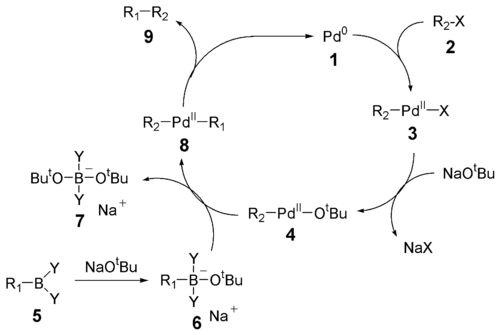
with vinyl
halides, while giving inversion
of stereochemistry with allylic and benzylic halides. The oxidative addition initially forms the cis-palladium complex, which rapidly isomerizes to the trans-complex.
derivative for the synthesis of caparratriene, a natural product that is highly active against leukemia:
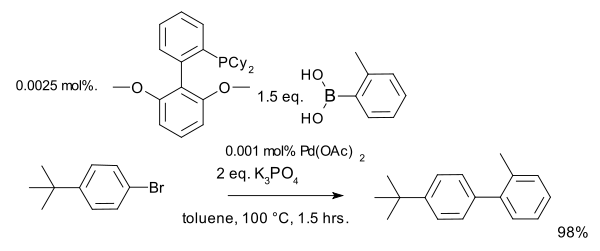
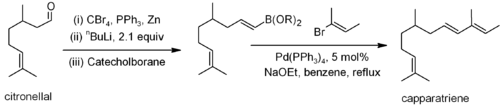 Recent applications of the Suzuki–Miyaura cross-coupling reaction in organic synthesis
Recent applications of the Suzuki–Miyaura cross-coupling reaction in organic synthesis
have been summarized by Kotha and co-workers. With a novel organophosphine ligand
(SPhos
), a catalyst loading of down to 0.001 mol% has been reported :
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
of an aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
- or vinyl
Vinyl
A vinyl compound is any organic compound that contains a vinyl group ,which are derivatives of ethene, CH2=CH2, with one hydrogen atom replaced with some other group...
-boronic acid
Boronic acid
A boronic acid is an alkyl or aryl substituted boric acid containing a carbon–boron bond belonging to the larger class of organoboranes. Boronic acids act as Lewis acids. Their unique feature is that they are capable of forming reversible covalent complexes with sugars, amino acids, hydroxamic...
with an aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
- or vinyl
Vinyl
A vinyl compound is any organic compound that contains a vinyl group ,which are derivatives of ethene, CH2=CH2, with one hydrogen atom replaced with some other group...
-halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
catalyzed by a palladium(0) complex
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
. It is widely used to synthesize
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
poly-olefins, styrenes, and substituted biphenyl
Biphenyl
Biphenyl is an organic compound that forms colorless crystals. It has a distinctively pleasant smell. Biphenyl is an aromatic hydrocarbon with a molecular formula 2...
s, and has been extended to incorporate alkyl bromides. Several reviews have been published.

Triflate
Trifluoromethanesulfonate, also known by the trivial name triflate, is a functional group with the formula CF3SO3-. The triflate group is often represented by -OTf, as opposed to -Tf...
s (OTf), instead of halides. Boronic esters and organotrifluoroborate salts
Organotrifluoroborate
Organotrifluoroborates are organoboron compounds that contain an anion with the general formula [RBF3]−. They can be thought of as protected boronic acids, or as adducts of carbanions and boron trifluoride. Organotrifluoroborates are tolerant of air and moisture and are easy to handle and purify...
may be used instead of boronic acids.
- Relative reactivity: R2-I > R2-OTf > R2-Br >> R2-Cl
First published in 1979 by Akira Suzuki
Akira Suzuki (chemist)
is a Japanese chemist and Nobel Prize Laureate , who first published the Suzuki reaction, the organic reaction of an aryl- or vinyl-boronic acid with an aryl- or vinyl-halide catalyzed by a palladium complex, in 1979.-Life:...
, the Suzuki reaction couples
Coupling reaction
A coupling reaction in organic chemistry is a catch-all term for a variety of reactions where two hydrocarbon fragments are coupled with the aid of a metal catalyst...
boronic acids (containing an organic part) to halides. The reaction relies on a palladium
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
catalyst such as tetrakis(triphenylphosphine)palladium(0)
Tetrakis(triphenylphosphine)palladium(0)
Tetrakispalladium is the chemical compound Pd[P3]4, often abbreviated Pd4, or even PdP4. It is a bright yellow crystalline solid that becomes brown upon decomposition in air.-Structure and properties:...
to effect part of the transformation. The palladium catalyst (more strictly a pre-catalyst) is 4-coordinate, and usually involves phosphine
Phosphine
Phosphine is the compound with the chemical formula PH3. It is a colorless, flammable, toxic gas. Pure phosphine is odourless, but technical grade samples have a highly unpleasant odor like garlic or rotting fish, due to the presence of substituted phosphine and diphosphine...
supporting groups.
The 2010 Nobel Prize in Chemistry
Nobel Prize in Chemistry
The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outstanding contributions in chemistry, physics, literature,...
was awarded to Suzuki for his discovery and development of this reaction. In many publications this reaction also goes by the name Suzuki-Miyaura reaction. It is also often referred to as "Suzuki Coupling".
Reaction mechanism
The mechanismReaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
of the Suzuki reaction is best viewed from the perspective of the palladium catalyst. The first step is the oxidative addition
Oxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre...
of palladium to the halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
2 to form the organopalladium
Organopalladium
Organopalladium chemistry is a branch of organometallic chemistry that deals with organic palladium compounds and their reactions. Palladium is often used as a catalyst in the reduction of alkenes and alkynes with hydrogen. This process involves the formation of a palladium-carbon covalent bond...
species 3. Reaction with base gives intermediate 4, which via transmetalation
Transmetalation
Transmetalation is a general chemical reaction type in organometallic chemistry describing the exchange of ligands between two metal centers....
with the boron-ate complex 6 forms the organopalladium
Organopalladium
Organopalladium chemistry is a branch of organometallic chemistry that deals with organic palladium compounds and their reactions. Palladium is often used as a catalyst in the reduction of alkenes and alkynes with hydrogen. This process involves the formation of a palladium-carbon covalent bond...
species 8. Reductive elimination of the desired product 9 restores the original palladium catalyst 1.

Oxidative addition
Oxidative addition proceeds with retention of stereochemistryStereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
with vinyl
Vinyl
A vinyl compound is any organic compound that contains a vinyl group ,which are derivatives of ethene, CH2=CH2, with one hydrogen atom replaced with some other group...
halides, while giving inversion
Walden inversion
Walden inversion is the inversion of a chiral center in a molecule in a chemical reaction. Since a molecule can form two enantiomers around a chiral center, the Walden inversion converts the configuration of the molecule from one enantiomeric form to the other. For example, in a SN2 reaction,...
of stereochemistry with allylic and benzylic halides. The oxidative addition initially forms the cis-palladium complex, which rapidly isomerizes to the trans-complex.
Reductive elimination
Using deuterium-labelling, Ridgway et al. have shown the reductive elimination proceeds with retention of stereochemistry. Relative reactivity of different metal complexes in the C-C reductive elimination was established: Pd(IV), Pd(II) > Pt(IV), Pt(II), Rh(III) > Ir(III), Ru(II), Os(II).Scope
The Suzuki coupling has been used on a citronellalCitronellal
Citronellal or rhodinal or 3,7-dimethyloct-6-en-1-al is a monoterpenoid, the main component in the mixture of terpenoid chemical compounds that give citronella oil its distinctive lemon scent....
derivative for the synthesis of caparratriene, a natural product that is highly active against leukemia:
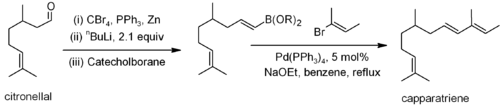
have been summarized by Kotha and co-workers. With a novel organophosphine ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
(SPhos
SPhos
SPhos is an organophosphorus compound derived from biphenyl. Its palladium complexes exhibit high activity for Suzuki coupling reactions involving aryl chlorides, which are unreactive with palladium complexes of most other phosphine ligands. The ligand has convenient handling characteristics since...
), a catalyst loading of down to 0.001 mol% has been reported :

See also
- Heck reactionHeck reactionThe Heck reaction is the chemical reaction of an unsaturated halide with an alkene and a base and palladium catalyst to form a substituted alkene. Together with the other palladium-catalyzed cross-coupling reactions, this reaction is of great importance, as it allows one to do substitution...
- Hiyama couplingHiyama couplingIn organic chemistry, a Hiyama coupling is a palladium or nickel-catalyzed cross coupling reaction of organosilanes with organic halides or triflates. Hiyama couplings were first reported by Yasuo Hatanaka and Tamejiro Hiyama in 1988....
- Kumada couplingKumada couplingA Kumada coupling or Kumada-Corriu coupling is a cross coupling reaction in organic chemistry between an alkyl or aryl Grignard reagent and an aryl or vinyl halocarbon catalysed by nickel or palladium. This reaction is relevant to organic synthesis because it gives access to styrene derivatives...
- Negishi couplingNegishi couplingThe Negishi coupling is a cross coupling reaction in organic chemistry involving an organozinc compound, an organic halide and a nickel or palladium catalyst creating a new carbon-carbon covalent bond:* The halide X can be chloride, bromine or iodine but also a triflate or acetyloxy group with as...
- Petasis reactionPetasis reactionThe Petasis reaction is the chemical reaction of an amine, aldehyde, and vinyl- or aryl-boronic acid to form substituted amines.Reported in 1993 by N.A. Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine, the Petasis reaction can be described as...
- Stille reactionStille reactionThe Stille reaction is a chemical reaction coupling an organotin compound with an sp2-hybridized organic halide catalyzed by palladium. The reaction is widely used in organic synthesis....
- Sonogashira couplingSonogashira couplingIn organic chemistry, a Sonogashira coupling is a coupling reaction of terminal alkynes with aryl or vinyl halides. This reaction was first reported by Kenkichi Sonogashira and Nobue Hagihara in 1975.-Catalyst:...

