2-Pyridone
Encyclopedia
2-Pyridone is an organic compound
with the formula . This colourless crystalline solid is used in peptide
synthesis. It is well known to form hydrogen bond
ed structures somewhat related to the base-pairing mechanism found in RNA
and DNA
. It is also a classic case of a molecule that exists as tautomer
s.
is the amide
group; a nitrogen
with a hydrogen
bound to it and a keto
group next to it. In peptide
s, amino acids are linked by this pattern, a feature responsible for some remarkable physical and chemical properties. In this and similar molecules, the hydrogen
bound to the nitrogen is suitable to form strong hydrogen bonds to other nitrogen
- and oxygen
-containing species.
 The hydrogen attached to the nitrogen can also move to the oxygen. Through movement of this hydrogen and electron
The hydrogen attached to the nitrogen can also move to the oxygen. Through movement of this hydrogen and electron
s, the second tautomer form
, 2-hydroxypyridine is formed. This lactam
lactim tautomer
ism can also be found in other molecules with a similar structure.
form is 2-pyridone. This has been confirmed by X-ray crystallography
which shows that the hydrogen in solid state is closer to the nitrogen than to the oxygen (because of the low electron density at the hydrogen the exact positioning is difficult), and IR-spectroscopy
, which shows that the C=O longitudinal frequency is present whilst the O-H frequencies are absent.
has been the subject of many publications. The energy difference appears to be very small and is dependent on the polarity
of the solvent
. Non-polar solvents
favour the formation of 2-hydroxypyridine whereas polar solvents
such as alcohols and water
favour the formation of 2-pyridone.
The energy difference for the two tautomers in the gas phase was measured by IR-spectroscopy
to be 2.43 to 3.3 kJ/mol
for the solid state and 8.95 kJ/mol and 8.83 kJ/mol for the liquid state.
transition state
and therefore has a high energy barrier for this tautomer
isation, which was calculated with theoretical methods
to be 125 or 210 kJ/mol. The direct tautomerisation is energetically not favoured. There are other possible mechanisms for this tautomerisation.
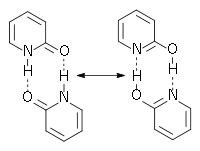 2-Pyridone and 2-hydroxypyridine can form dimers with two hydrogen bonds.
2-Pyridone and 2-hydroxypyridine can form dimers with two hydrogen bonds.
.
In the solid state the hydrogen is located closer to the oxygen so it could be considered to be right to call the colourless crystals in the flask 2-pyridone.
is formed. Hydrophobic effects in non-polar solvents
lead to a predominance of the dimer. The ratio of the tautomeric forms is also dependent on the solvent. All possible tautomers and dimers can be present and form an equilibrium, and the exact measurement of all the equilibrium constants in the system is extremely difficult.
(NMR-spectroscopy
is a slow method, high resolution IR-spectroscopy
in solvent is difficult, the broad absorption in UV-spectroscopy makes it hard to discriminate 3 and more very similar molecules).
Some publications only focus one of the two possible patterns, and neglect the influence of the other. For example, to calculation of the energy difference of the two tautomers in a non-polar solution will lead to a wrong result if a large quantity of the substance is on the side of the dimer in an equilibrium.
of the dimer is a self catalytic path from one tautomer to the other. Protic solvents also mediate the proton transfer during the tautomerisation.
:
Pyridine forms an N-oxide
with some oxidation agents such as hydrogen peroxide
. This pyridine-N-oxide
undergoes a rearrangement reaction to 2-pyridone in acetic anhydride
:
In the Guareschi-Thorpe condensation cyanoacetamide reacts with a 1,3-diketone
to a 2-pyridone
. The reaction is named after Icilio Guareschi
and Jocelyn Field Thorpe
.
of sugars and that 2-pyridone has a large effect on the reaction from activated esters with amine
s in nonpolar solvent
, which is attributed to its tautomerisation and ability to as a ditopic receptor. Current interest focuses on proton transfer from 2-pyridone and its tautomer, using isotope labeling, kinetics
and quantum chemical methods were used on the mechanism to determine the rate determining step in the reaction.
or RNA
. These dimers are sometimes used as simple models for base pair
s in experiment
al and theoretical studies
.
serve as ligands in coordination chemistry, usually as a 1,3-bridging ligand akin to carboxylate
.
s.
crystallopoietes, a member of the phylum, Actinobacteria
, which includes numerous related organisms that have been shown to degrade pyridine or one or more alkyl-, carboxyl-, or hydroxyl-substituted pyridines. 2-Pyridone degradation is commonly initiated by mono-oxygenase attack, resulting in a diol, such as 2,5-dihydroxypyridine, which is metabolized via the maleamate pathway. Fission of the ring proceeds via action of 2,5-dihydroxypyridine monooxygenase, which is also involved in metabolism of nicotinic acid via the maleamate pathway. In the case of Arthrobacter crystallopoietes, at least part of the degradative pathway is plasmid-borne. Pyridine diols undergo chemical transformation in solution to form intensely colored pigments. Similar pigments have been observed in quinoline
degradation, also owing to transformation of metabolites, however the yellow pigments often reported in degradation of many pyridine solvents, such as unsubstituted pyridine
or picoline
, generally result from overproduction of riboflavin
in the presence of these solvents. Generally speaking, degradation of pyridones, dihydroxypyridines, and pyridinecarboxylic acids is commonly mediated by oxygenases, whereas degradation of pyridine solvents often is not, and may in some cases involve an initial reductive step.
(m), 1682 (s), 1649 (vs), 1609 (vs), 1578 (vs), 1540 (s), 1456 (m), 1433 (m), 1364 (w), 1243 (m), 1156 (m), 1098 (m), 983 (m), 926 (w), 781 (s), 730 (w), 612 (w), 560 (w), 554 (w), 526 (m), 476 (m), 451 (w).
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
with the formula . This colourless crystalline solid is used in peptide
Peptide
Peptides are short polymers of amino acid monomers linked by peptide bonds. They are distinguished from proteins on the basis of size, typically containing less than 50 monomer units. The shortest peptides are dipeptides, consisting of two amino acids joined by a single peptide bond...
synthesis. It is well known to form hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
ed structures somewhat related to the base-pairing mechanism found in RNA
RNA
Ribonucleic acid , or RNA, is one of the three major macromolecules that are essential for all known forms of life....
and DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
. It is also a classic case of a molecule that exists as tautomer
Tautomer
Tautomers are isomers of organic compounds that readily interconvert by a chemical reaction called tautomerization. This reaction commonly results in the formal migration of a hydrogen atom or proton, accompanied by a switch of a single bond and adjacent double bond...
s.
Structure
The most prominent feature of 2-pyridone2-Pyridone
2-Pyridone is an organic compound with the formula . This colourless crystalline solid is used in peptide synthesis. It is well known to form hydrogen bonded structures somewhat related to the base-pairing mechanism found in RNA and DNA...
is the amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
group; a nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
with a hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
bound to it and a keto
Keto
Keto can refer to:* The Keto people, an ethnic group of the Siberian North.* Ceto or Keto, a sea goddess in Greek mythology.* Ketone or keto group, the functional group in the chemical compounds ketones....
group next to it. In peptide
Peptide
Peptides are short polymers of amino acid monomers linked by peptide bonds. They are distinguished from proteins on the basis of size, typically containing less than 50 monomer units. The shortest peptides are dipeptides, consisting of two amino acids joined by a single peptide bond...
s, amino acids are linked by this pattern, a feature responsible for some remarkable physical and chemical properties. In this and similar molecules, the hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
bound to the nitrogen is suitable to form strong hydrogen bonds to other nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
- and oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
-containing species.
Tautomerism

Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s, the second tautomer form
Tautomer
Tautomers are isomers of organic compounds that readily interconvert by a chemical reaction called tautomerization. This reaction commonly results in the formal migration of a hydrogen atom or proton, accompanied by a switch of a single bond and adjacent double bond...
, 2-hydroxypyridine is formed. This lactam
Lactam
A lactam is a cyclic amide. Prefixes indicate how many carbon atoms are present in the ring: β-lactam , γ-lactam , δ-lactam...
lactim tautomer
Tautomer
Tautomers are isomers of organic compounds that readily interconvert by a chemical reaction called tautomerization. This reaction commonly results in the formal migration of a hydrogen atom or proton, accompanied by a switch of a single bond and adjacent double bond...
ism can also be found in other molecules with a similar structure.
Tautomerism in the solid state
The predominant solid stateSolid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
form is 2-pyridone. This has been confirmed by X-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
which shows that the hydrogen in solid state is closer to the nitrogen than to the oxygen (because of the low electron density at the hydrogen the exact positioning is difficult), and IR-spectroscopy
Infrared spectroscopy
Infrared spectroscopy is the spectroscopy that deals with the infrared region of the electromagnetic spectrum, that is light with a longer wavelength and lower frequency than visible light. It covers a range of techniques, mostly based on absorption spectroscopy. As with all spectroscopic...
, which shows that the C=O longitudinal frequency is present whilst the O-H frequencies are absent.
Tautomerism in solution
The determination of which of the two tautomeric forms is present in solutionSolution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
has been the subject of many publications. The energy difference appears to be very small and is dependent on the polarity
Chemical polarity
In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in...
of the solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
. Non-polar solvents
Chemical polarity
In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in...
favour the formation of 2-hydroxypyridine whereas polar solvents
Chemical polarity
In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in...
such as alcohols and water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
favour the formation of 2-pyridone.
The energy difference for the two tautomers in the gas phase was measured by IR-spectroscopy
Infrared spectroscopy
Infrared spectroscopy is the spectroscopy that deals with the infrared region of the electromagnetic spectrum, that is light with a longer wavelength and lower frequency than visible light. It covers a range of techniques, mostly based on absorption spectroscopy. As with all spectroscopic...
to be 2.43 to 3.3 kJ/mol
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
for the solid state and 8.95 kJ/mol and 8.83 kJ/mol for the liquid state.
Tautomerisation mechanism A
The single molecular tautomerisation has a forbidden 1-3 suprafacialRearrangement reaction
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
transition state
Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
and therefore has a high energy barrier for this tautomer
Tautomer
Tautomers are isomers of organic compounds that readily interconvert by a chemical reaction called tautomerization. This reaction commonly results in the formal migration of a hydrogen atom or proton, accompanied by a switch of a single bond and adjacent double bond...
isation, which was calculated with theoretical methods
Theoretical chemistry
Theoretical chemistry seeks to provide theories that explain chemical observations. Often, it uses mathematical and computational methods that, at times, require advanced knowledge. Quantum chemistry, the application of quantum mechanics to the understanding of valency, is a major component of...
to be 125 or 210 kJ/mol. The direct tautomerisation is energetically not favoured. There are other possible mechanisms for this tautomerisation.
Dimerisation

Aggregation in the solid state
In the solid state the dimeric form is not present; the 2-pyridones form a helical structure over hydrogen bonds. Some substituted 2-pyridones form the dimer in solid state, for example the 5-methyl-3-carbonitrile-2-pyridone. The determination of all these structures was done by X-ray crystallographyX-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
.
In the solid state the hydrogen is located closer to the oxygen so it could be considered to be right to call the colourless crystals in the flask 2-pyridone.
Aggregation in solution
In solution the dimeric form is present; the ratio of dimerisation is strongly dependent on the polarity of the solvent. Polar and protic solvents interact with the hydrogen bonds and more monomerMonomer
A monomer is an atom or a small molecule that may bind chemically to other monomers to form a polymer; the term "monomeric protein" may also be used to describe one of the proteins making up a multiprotein complex...
is formed. Hydrophobic effects in non-polar solvents
Chemical polarity
In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in...
lead to a predominance of the dimer. The ratio of the tautomeric forms is also dependent on the solvent. All possible tautomers and dimers can be present and form an equilibrium, and the exact measurement of all the equilibrium constants in the system is extremely difficult.
(NMR-spectroscopy
NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy, is a research technique that exploits the magnetic properties of certain atomic nuclei to determine physical and chemical properties of atoms or the molecules in which they are contained...
is a slow method, high resolution IR-spectroscopy
Infrared spectroscopy
Infrared spectroscopy is the spectroscopy that deals with the infrared region of the electromagnetic spectrum, that is light with a longer wavelength and lower frequency than visible light. It covers a range of techniques, mostly based on absorption spectroscopy. As with all spectroscopic...
in solvent is difficult, the broad absorption in UV-spectroscopy makes it hard to discriminate 3 and more very similar molecules).
Some publications only focus one of the two possible patterns, and neglect the influence of the other. For example, to calculation of the energy difference of the two tautomers in a non-polar solution will lead to a wrong result if a large quantity of the substance is on the side of the dimer in an equilibrium.
Tautomerisation mechanism B
The direct tautomerisation is not energetically favoured, but a dimerisation followed by a double proton transfer and dissociationDissociation (chemistry)
Dissociation in chemistry and biochemistry is a general process in which ionic compounds separate or split into smaller particles, ions, or radicals, usually in a reversible manner...
of the dimer is a self catalytic path from one tautomer to the other. Protic solvents also mediate the proton transfer during the tautomerisation.
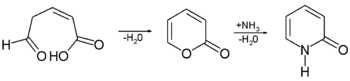
Synthesis
2-Pyrone can be obtained by a cyclisation reaction, and converted to 2-pyridone via an exchange reaction with ammoniaAmmonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
:
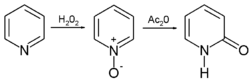
Pyridine forms an N-oxide
Amine oxide
An amine oxide, also known as amine-N-oxide and N-oxide, is a chemical compound that contains the functional group R3N+-O−, an N-O bond with three additional hydrogen and/or hydrocarbon side chains attached to N. Sometimes it is written as R3N→O or, wrongly, as R3N=O.In the strict sense the...
with some oxidation agents such as hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
. This pyridine-N-oxide
Amine oxide
An amine oxide, also known as amine-N-oxide and N-oxide, is a chemical compound that contains the functional group R3N+-O−, an N-O bond with three additional hydrogen and/or hydrocarbon side chains attached to N. Sometimes it is written as R3N→O or, wrongly, as R3N=O.In the strict sense the...
undergoes a rearrangement reaction to 2-pyridone in acetic anhydride
Acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula 2O. Commonly abbreviated Ac2O, it is the simplest isolatable acid anhydride and is a widely used reagent in organic synthesis...
:
In the Guareschi-Thorpe condensation cyanoacetamide reacts with a 1,3-diketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
to a 2-pyridone
2-Pyridone
2-Pyridone is an organic compound with the formula . This colourless crystalline solid is used in peptide synthesis. It is well known to form hydrogen bonded structures somewhat related to the base-pairing mechanism found in RNA and DNA...
. The reaction is named after Icilio Guareschi
Icilio Guareschi
Icilio Guareschi was an Italian chemist.Icilio Guareschi studied at the University of Bologna and got his Ph.D there in 1871...
and Jocelyn Field Thorpe
Jocelyn Field Thorpe
Sir Jocelyn Field Thorpe FRS was an English chemist who discovered the Thorpe reaction and the Thorpe-Ingold effect....
.
Catalytic activity
2-Pyridone catalyses a variety of proton-dependent reactions, for example the aminolysis of esters. In some cases, molten 2-pyridone is used as a solvent. The mutarotationMutarotation
Mutarotation is the change in the optical rotation that occurs by epimerization...
of sugars and that 2-pyridone has a large effect on the reaction from activated esters with amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s in nonpolar solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
, which is attributed to its tautomerisation and ability to as a ditopic receptor. Current interest focuses on proton transfer from 2-pyridone and its tautomer, using isotope labeling, kinetics
Chemical kinetics
Chemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
and quantum chemical methods were used on the mechanism to determine the rate determining step in the reaction.
Molecular recognition
The structures shown in the figure above are reminiscent of the base pairs found in the DNADNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
or RNA
RNA
Ribonucleic acid , or RNA, is one of the three major macromolecules that are essential for all known forms of life....
. These dimers are sometimes used as simple models for base pair
Base pair
In molecular biology and genetics, the linking between two nitrogenous bases on opposite complementary DNA or certain types of RNA strands that are connected via hydrogen bonds is called a base pair...
s in experiment
Experiment
An experiment is a methodical procedure carried out with the goal of verifying, falsifying, or establishing the validity of a hypothesis. Experiments vary greatly in their goal and scale, but always rely on repeatable procedure and logical analysis of the results...
al and theoretical studies
Theoretical chemistry
Theoretical chemistry seeks to provide theories that explain chemical observations. Often, it uses mathematical and computational methods that, at times, require advanced knowledge. Quantum chemistry, the application of quantum mechanics to the understanding of valency, is a major component of...
.
Coordination chemistry
2-Pyridone and some derivativesDerivative (chemistry)
In chemistry, a derivative is a compound that is derived from a similar compound by some chemical or physical process. In the past it was also used to mean a compound that can be imagined to arise from another compound, if one atom is replaced with another atom or group of atoms, but modern...
serve as ligands in coordination chemistry, usually as a 1,3-bridging ligand akin to carboxylate
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
.
In nature
2-Pyridone is not naturally occurring, but a derivative has been isolated as a cofactor in certain hydrogenaseHydrogenase
A hydrogenase is an enzyme that catalyses the reversible oxidation of molecular hydrogen . Hydrogenases play a vital role in anaerobic metabolism....
s.
Environmental Behavior
2-Pyridone is rapidly degraded by microorganisms in the soil environment, with a half life less than one week. Organisms capable of growth on 2-pyridone as a sole source of carbon, nitrogen, and energy have been isolated by a number of researchers. The most extensively studied 2-pyridone degrader is the gram positive bacterium, ArthrobacterArthrobacter
Arthrobacter is a genus of bacteria that is commonly found in soil. All species in this genus are Gram-positive obligate aerobes that are rods during exponential growth and cocci in their stationary phase....
crystallopoietes, a member of the phylum, Actinobacteria
Actinobacteria
Actinobacteria are a group of Gram-positive bacteria with high guanine and cytosine content. They can be terrestrial or aquatic. Actinobacteria is one of the dominant phyla of the bacteria....
, which includes numerous related organisms that have been shown to degrade pyridine or one or more alkyl-, carboxyl-, or hydroxyl-substituted pyridines. 2-Pyridone degradation is commonly initiated by mono-oxygenase attack, resulting in a diol, such as 2,5-dihydroxypyridine, which is metabolized via the maleamate pathway. Fission of the ring proceeds via action of 2,5-dihydroxypyridine monooxygenase, which is also involved in metabolism of nicotinic acid via the maleamate pathway. In the case of Arthrobacter crystallopoietes, at least part of the degradative pathway is plasmid-borne. Pyridine diols undergo chemical transformation in solution to form intensely colored pigments. Similar pigments have been observed in quinoline
Quinoline
Quinoline is a heterocyclic aromatic organic compound. It has the formula C9H7N and is a colourless hygroscopic liquid with a strong odour. Aged samples, if exposed to light, become yellow and later brown...
degradation, also owing to transformation of metabolites, however the yellow pigments often reported in degradation of many pyridine solvents, such as unsubstituted pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
or picoline
Picoline
Picoline refers to three different methylpyridine isomers, all with the chemical formula C6H7N and a molar mass of 93.13 g mol−1. All three are colourless liquids at room temperature and pressure and are miscible with water and most organic solvents...
, generally result from overproduction of riboflavin
Riboflavin
Riboflavin, also known as vitamin B2 or additive E101, is an easily absorbed micronutrient with a key role in maintaining health in humans and animals. It is the central component of the cofactors FAD and FMN, and is therefore required by all flavoproteins. As such, vitamin B2 is required for a...
in the presence of these solvents. Generally speaking, degradation of pyridones, dihydroxypyridines, and pyridinecarboxylic acids is commonly mediated by oxygenases, whereas degradation of pyridine solvents often is not, and may in some cases involve an initial reductive step.
1H-NMR
1H-NMR (400 MHz, CD3OD): /ρ = 8.07 (dd,3J = 2.5 Hz,4J = 1.1 Hz, 1H, C-6), 7.98 (dd,3J = 4.0 Hz,3J = 2.0 Hz, 1H, C-3), 7.23 (dd,3J = 2.5 Hz,3J = 2.0 Hz, 1H, C-5), 7.21 (dd,3J = 4.0 Hz,4J = 1.0 Hz, 1H, C-4).13C-NMR
(100.57 MHz, CD3OD): ρ = 155.9 (C-2), 140.8 (C-4), 138.3 (C-6), 125.8 (C-3), 124.4 (C-5)IR spectroscopy
(KBr): ν = 3440 cm−1–1 (br, m), 3119 (m), 3072 (m), 2986(m), 1682 (s), 1649 (vs), 1609 (vs), 1578 (vs), 1540 (s), 1456 (m), 1433 (m), 1364 (w), 1243 (m), 1156 (m), 1098 (m), 983 (m), 926 (w), 781 (s), 730 (w), 612 (w), 560 (w), 554 (w), 526 (m), 476 (m), 451 (w).