Sakurai reaction
Encyclopedia
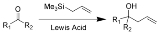
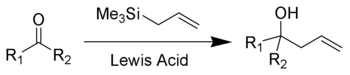
The Sakurai reaction is the chemical reaction
of carbon
electrophile
s (such as a ketone
shown here) with allylic silane
s catalyzed by strong Lewis acid
s. It is named after the chemists Akira Hosomi and Hideki Sakurai
.
 Lewis acid activation is essential for complete reaction. Strong Lewis acids such as titanium tetrachloride
Lewis acid activation is essential for complete reaction. Strong Lewis acids such as titanium tetrachloride
, boron trifluoride
, tin tetrachloride, and AlCl(Et)2 are all effective in promoting the Hosomi reaction. The reaction is a type of electrophilic allyl shift with formation of an intermediate beta-silyl carbocation. Driving force is the stabilization of said carbocation by the beta-silicon effect
.
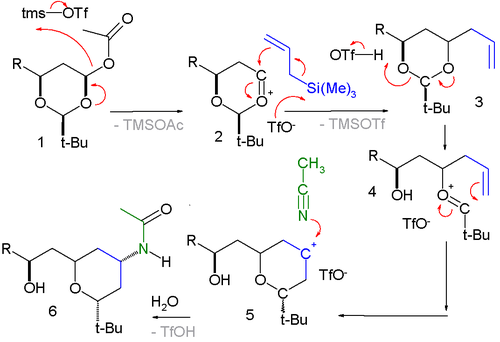
The reaction has been applied in a Sakurai-Prins-Ritter multicomponent reaction with in step two a Prins reaction
and in step three a Ritter reaction
:
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
of carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
electrophile
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
s (such as a ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
shown here) with allylic silane
Silane
Silane is a toxic, extremely flammable chemical compound with chemical formula SiH4. In 1857, the German chemists and Friedrich Woehler discovered silane among the products formed by the action of hydrochloric acid on aluminum silicide, which they had previously prepared...
s catalyzed by strong Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
s. It is named after the chemists Akira Hosomi and Hideki Sakurai
Hideki Sakurai
is a Japanese chemistHe discovered the Sakurai reaction in 1976.-References:...
.

Titanium tetrachloride
Titanium tetrachloride is the inorganic compound with the formula TiCl4. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. TiCl4 is an unusual example of a metal halide that is highly volatile...
, boron trifluoride
Boron trifluoride
Boron trifluoride is the chemical compound with the formula BF3. This pungent colourless toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.-Structure and bonding:...
, tin tetrachloride, and AlCl(Et)2 are all effective in promoting the Hosomi reaction. The reaction is a type of electrophilic allyl shift with formation of an intermediate beta-silyl carbocation. Driving force is the stabilization of said carbocation by the beta-silicon effect
Beta-silicon effect
The beta-silicon effect also called silicon hyperconjugation in organosilicon chemistry is a special type of hyperconjugation and describes the stabilizing effect of a silicon atom placed in a position one removed from a carbocation. A prerequisite is an antiperiplanar relationship between the two...
.
The reaction has been applied in a Sakurai-Prins-Ritter multicomponent reaction with in step two a Prins reaction
Prins reaction
The Prins reaction is an organic reaction consisting of an electrophilic addition of an aldehyde or ketone to an alkene or alkyne followed by capture of a nucleophile. The outcome of the reaction depends on reaction conditions . With water and a protic acid such as sulfuric acid as the reaction...
and in step three a Ritter reaction
Ritter reaction
The Ritter reaction is a chemical reaction that transforms a nitrile into a N-alkyl amide using various alkylating reagents, for example, strong acid and isobutylene....
:

Various Reactions
The Hosomi-Sakurai reaction can be performed on a number of functional groups. An electrophilic carbon, activated by a Lewis Acid, is required. Below is a list of three different functional groups that can be used in the Hosomi-Sakurai reaction.Mechanism
The proposed mechanism for the Hosomi-Sakurai reaction using a ketone is displayed above. The mechanisms for all Hosomi-Sakurai reactions follow the same general principle where a strong Lewis Acid activates an electrophilic carbon, which then undergoes nucleophilic attack from electrons on the allylic silane.β-Silicon Effect Stabilization
As displayed in the mechanism, the Hosomi-Sakurai reaction goes through a secondary carbocation intermediate. Secondary carbonations are inherently unstable, however the β-silicon effect from the silicon atom stabilizes the carbocation. Silicon is able to donate into an empty p-orbital, and the silicon orbital is shared between the two carbons. This stabilizes the positive charge over 3 orbitals. Another term for the β-silicon effect is silicon-hyperconjugation. This interaction is essential for the reaction to go to completion.External links
- Hosomi-Sakurai reaction @ www.organic-chemistry.org Link
- Akira Hosomi HP

