Carbenoid
Encyclopedia
In chemistry
a carbenoid is a reactive intermediate
that shares reaction characteristics with a carbene
. In the Simmons-Smith reaction
the carbenoid intermediate is a zinc
/ iodine
complex
that takes the form of
This complex reacts with an alkene
to form a cyclopropane
just as a carbene would do.
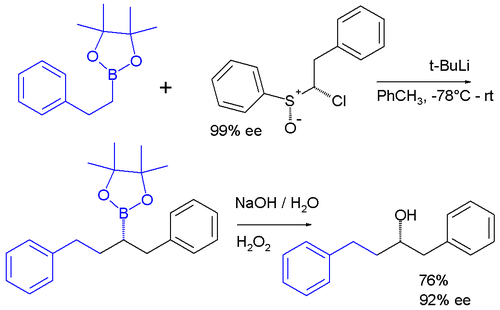
Carbenoids appear as intermediates in many other reactions. In one system a carbenoid chloroalkyllithium reagent is prepared in situ from a sulfoxide
and t-BuLi which reacts the boronic ester to give an ate complex. The ate complex undergoes a 1,2-metallate rearrangement to give the homologated product, which is then further oxidise to a secondary alcohol.
The enantiopurity of the chiral sulfoxide is preserved in the ultimate product after oxidation of the boronic ester to the alcohol
indicating that a true carbene was never involved in the sequence.
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
a carbenoid is a reactive intermediate
Reactive intermediate
In chemistry a reactive intermediate is a short-lived, high energy, highly reactive molecule. When generated in a chemical reaction it will quickly convert into a more stable molecule. Only in exceptional cases can these compounds be isolated and stored, e.g. low temperatures, matrix isolation...
that shares reaction characteristics with a carbene
Carbene
In chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
. In the Simmons-Smith reaction
Simmons-Smith reaction
The Simmons–Smith reaction is an organic cheletropic reaction in which a carbenoid reacts with an alkene to form a cyclopropane. It is named after Howard Ensign Simmons, Jr. and R. D. Smith...
the carbenoid intermediate is a zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
/ iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
complex
Complex (chemistry)
In chemistry, a coordination complex or metal complex, is an atom or ion , bonded to a surrounding array of molecules or anions, that are in turn known as ligands or complexing agents...
that takes the form of
- I-CH2-Zn-I
This complex reacts with an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
to form a cyclopropane
Cyclopropane
Cyclopropane is a cycloalkane molecule with the molecular formula C3H6, consisting of three carbon atoms linked to each other to form a ring, with each carbon atom bearing two hydrogen atoms...
just as a carbene would do.
Carbenoids appear as intermediates in many other reactions. In one system a carbenoid chloroalkyllithium reagent is prepared in situ from a sulfoxide
Sulfoxide
A sulfoxide is a chemical compound containing a sulfinyl functional group attached to two carbon atoms. Sulfoxides can be considered as oxidized sulfides...
and t-BuLi which reacts the boronic ester to give an ate complex. The ate complex undergoes a 1,2-metallate rearrangement to give the homologated product, which is then further oxidise to a secondary alcohol.
The enantiopurity of the chiral sulfoxide is preserved in the ultimate product after oxidation of the boronic ester to the alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
indicating that a true carbene was never involved in the sequence.