Organosilicon
Encyclopedia
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
compounds
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
containing carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
silicon
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
bonds
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
. Organosilicon chemistry is the corresponding science exploring their properties and reactivity.
Like carbon, the organically bound silicon is tetravalent and tetrahedral
Tetrahedral molecular geometry
In a tetrahedral molecular geometry a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are cos−1 ≈ 109.5° when all four substituents are the same, as in CH4. This molecular geometry is common throughout the first...
. Carbon-silicon bonds are generally absent in biochemical
Biochemistry
Biochemistry, sometimes called biological chemistry, is the study of chemical processes in living organisms, including, but not limited to, living matter. Biochemistry governs all living organisms and living processes...
processes, although there are reports of their fleeting existence in a freshwater alga. The first organosilicon compound, tetraethylsilane was discovered by Charles Friedel
Charles Friedel
Charles Friedel was a French chemist and mineralogist. A native of Strasbourg, France, he was a student of Louis Pasteur at the Sorbonne...
and James Crafts
James Crafts
James Mason Crafts was an American chemist, best known for developing the Friedel-Crafts alkylation and acylation reactions with Charles Friedel in 1876.-Biography:...
in 1863 by reaction of tetrachlorosilane with diethylzinc
Diethylzinc
Diethylzinc 2Zn, or DEZn, is a highly pyrophoric organozinc compound consisting of a zinc center bound to two ethyl groups. This colourless liquid is an important reagent in organic chemistry and available commercially as a solution in hexanes, heptane, or toluene.-Synthesis:Edward Frankland first...
. The carbosilicon silicon carbide
Silicon carbide
Silicon carbide , also known as carborundum, is a compound of silicon and carbon with chemical formula SiC. It occurs in nature as the extremely rare mineral moissanite. Silicon carbide powder has been mass-produced since 1893 for use as an abrasive...
is an inorganic
Inorganic chemistry
Inorganic chemistry is the branch of chemistry concerned with the properties and behavior of inorganic compounds. This field covers all chemical compounds except the myriad organic compounds , which are the subjects of organic chemistry...
compound.
Organosilanes
Carbon–silicon bonds compared to carbon–carbon bonds are longer (186 pm vs. 154 pm) and weaker with bond dissociation energyBond dissociation energy
In chemistry, bond-dissociation energy or D0, is one measure of the bond strength in a chemical bond. It is defined as the standard enthalpy change when a bond is cleaved by homolysis, with reactants and products of the homolysis reaction at 0 K...
451 kJ/mol
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
vs. 607 kJ/mol . The C–Si is somewhat polarized towards carbon due to its higher electronegativity
Electronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
(C 2.55 vs Si 1.90). One manifestation of bond polarization in organosilanes is found in the Sakurai reaction
Sakurai reaction
The Sakurai reaction is the chemical reaction of carbon electrophiles with allylic silanes catalyzed by strong Lewis acids. It is named after the chemists Akira Hosomi and Hideki Sakurai.Lewis acid activation is essential for complete reaction...
. In oxidative couplings silicon is represented by the Hiyama coupling
Hiyama coupling
In organic chemistry, a Hiyama coupling is a palladium or nickel-catalyzed cross coupling reaction of organosilanes with organic halides or triflates. Hiyama couplings were first reported by Yasuo Hatanaka and Tamejiro Hiyama in 1988....
. Certain alkyl silanes can be oxidized to an alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
in the Fleming–Tamao oxidation.
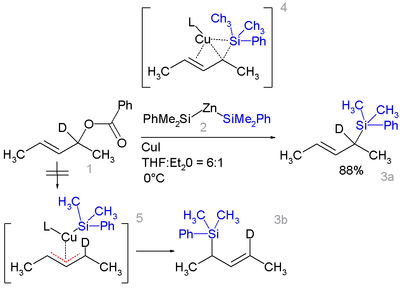
Certain allyl
Allyl
An allyl group is a substituent with the structural formula H2C=CH-CH2R, where R is the connection to the rest of the molecule. It is made up of a methylene , attached to a vinyl group . The name is derived from the Latin word for garlic, Allium sativum. Theodor Wertheim isolated an allyl...
silanes can be prepared from allylic ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
such as 1 and monosilylcopper compounds such as 2 in .
In this reaction type silicon polarity is reversed in a chemical bond with zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
and a formal allylic substitution on the benzoyl
Benzoyl
In organic chemistry, benzoyl is the acyl of benzoic acid, with structure C6H5CO-. It should not be confused with benzyl, which is the radical or ion formed from the removal of one of the methyl hydrogens of toluene...
oxy group takes place.
The chemistry of silane
Silane
Silane is a toxic, extremely flammable chemical compound with chemical formula SiH4. In 1857, the German chemists and Friedrich Woehler discovered silane among the products formed by the action of hydrochloric acid on aluminum silicide, which they had previously prepared...
s such as tetramethylsilane
Tetramethylsilane
Tetramethylsilane is the chemical compound with the formula Si4. It is the simplest tetraorganosilane. Like all silanes, the TMS framework is tetrahedral...
is comparable to that of alkane
Alkane
Alkanes are chemical compounds that consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds without any cycles...
s in many aspects such as thermal stability. The β-silicon effect
Beta-silicon effect
The beta-silicon effect also called silicon hyperconjugation in organosilicon chemistry is a special type of hyperconjugation and describes the stabilizing effect of a silicon atom placed in a position one removed from a carbocation. A prerequisite is an antiperiplanar relationship between the two...
describes the stabilizing effect of a β-silicon atom on a carbocation with many implications for reactivity.
Siloxides
More notably bonds of silicon to oxygenOxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
are much shorter and stronger (809 compared to 538 kJ/mol) than that of those of carbon to oxygen. The polarization in this bond increases towards oxygen. Examples are silyl acetals RR'Si(OR)2, the silanol
Silanol
Silanol, also known as silyl alcohol, is a chemical with formula SiH3OH. It is the simplest silicon alcohol, and is a heavy, volatile, colorless, flammable liquid. At room temperature it is a polar liquid...
s, the siloxane
Siloxane
A siloxane is any chemical compound composed of units of the form R2SiO, where R is a hydrogen atom or a hydrocarbon group. They belong to the wider class of organosilicon compounds....
s and the polymeric polysiloxanes. Silyl ether
Silyl ether
Silyl ethers are a group of chemical compounds which contain a silicon atom covalently bonded to an alkoxy group. The general structure is R1R2R3Si−O−R4 where R4 is an alkyl group or an aryl group. Silyl ethers are usually used as protecting groups for alcohols in organic synthesis...
s are extensively used as protective groups for alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s. Only silicon bonds to fluorine
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
are stronger and that is why the fluorine source TASF
TASF reagent
The TASF reagent or trissulfonium difluorotrimethylsilicate is a reagent in organic chemistry with structural formula [3S]+[F2Si3]-. It is an anhydrous source of fluoride and is used to cleave silyl ether protective groups. Many other fluoride reagents are known, but few are truly anhydrous,...
(or more commonly TBAF
Tetra-n-butylammonium fluoride
Tetra-n-butylammonium fluoride or TBAF is a quaternary ammonium salt with the chemical formula 4N+F-. It is commercially available as the trihydrate and as a solution in tetrahydrofuran....
) is useful in deprotection. The favorable formation of Si–O bonds drive many organic reaction
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
s such as the Brook rearrangement
Brook rearrangement
The Brook rearrangement in organic chemistry is a rearrangement reaction in which a organosilyl group switches position with a hydroxyl proton over a carbon to oxygen covalent bond under the influence of a base . It is named for the Canadian chemist Adrian Gibbs Brook...
and Peterson olefination
Peterson olefination
The Peterson olefination is the chemical reaction of α-silyl carbanions 1 with ketones to form a β-hydroxysilane 2 which eliminates to form alkenes 3.Several reviews have been published....
.
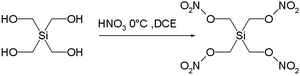
Another manifestation is the highly explosive nature of the silicon pendant Si(CH2ONO2)4 and Si(CH2N3)4 of pentaerythritol tetranitrate :
A single crystal of this compound, first synthesized in 2007 even detonates when in contact with a teflon spatula and in fact made full characterization impossible. Another contributor to its exothermic decomposition (inferred from much safer in silico
In silico
In silico is an expression used to mean "performed on computer or via computer simulation." The phrase was coined in 1989 as an analogy to the Latin phrases in vivo and in vitro which are commonly used in biology and refer to experiments done in living organisms and outside of living organisms,...
experimentation) is the ability of silicon in its crystal phase to coordinate to two oxygen nitrito groups in addition to regular coordination to the four carbon atoms. This additional coordination would make formation of silicon dioxide
Silicon dioxide
The chemical compound silicon dioxide, also known as silica , is an oxide of silicon with the chemical formula '. It has been known for its hardness since antiquity...
(one of the decomposition products) more facile.
Silyl halides
Organosilyl halides are important reagentReagent
A reagent is a "substance or compound that is added to a system in order to bring about a chemical reaction, or added to see if a reaction occurs." Although the terms reactant and reagent are often used interchangeably, a reactant is less specifically a "substance that is consumed in the course of...
s in organic chemistry notably trimethylsilyl chloride
Trimethylsilyl chloride
Trimethylsilyl chloride, also known as chlorotrimethylsilane is a silyl halide, with a variety of different uses in chemistry. It has the formula 3SiCl, and under standard conditions it is a colourless liquid, which is stable in the absence of water...
Me3SiCl. A classic method called the Flood reaction for the synthesis of this compound class is by heating hexaalkyldisiloxanes R3SiOSiR3 with concentrated sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
and a sodium halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
. Other relevant silyl halides are dichloromethylphenylsilane, dimethyldichlorosilane, methyltrichlorosilane, (4-aminobutyl)diethoxymethylsilane, trichloro(chloromethyl)silane, trichloro(dichlorophenyl)silane, trichloroethylsilane, trichlorophenylsilane and trimethylchlorosilane
Silyl hydrides
The silicon to hydrogen bond is longer than the C–H bond (148 compared to 105 pm) and weaker (299 compared to 338 kJ/mol). Hydrogen is more electronegative than silicon hence the naming convention of silyl hydrides. The parent compound SiH4 is called silaneSilane
Silane is a toxic, extremely flammable chemical compound with chemical formula SiH4. In 1857, the German chemists and Friedrich Woehler discovered silane among the products formed by the action of hydrochloric acid on aluminum silicide, which they had previously prepared...
, and an example is phenylsilane
Phenylsilane
Phenylsilane, also known as silylbenzene, a colorless liquid, is one of the simplest organosilanes with the formula C6H5SiH3. It is structurally related to toluene, with a silyl group replacing the methyl group. Both of these compounds have similar densities and boiling points due to these...
. Silyl hydrides are very reactive and used as reducing agent
Reducing agent
A reducing agent is the element or compound in a reduction-oxidation reaction that donates an electron to another species; however, since the reducer loses an electron we say it is "oxidized"...
s for example PMHS
PMHS
Polymethylhydrosiloxane is a polymer with the general structure --. It is used in organic chemistry as a mild and stable reducing agent easily transferring hydrides to metal centers....
.
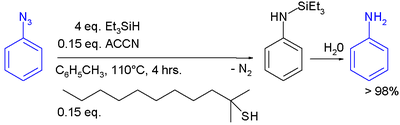
In one study triethylsilylhydride is used in the conversion of an phenyl azide
Phenyl azide
Phenylazide is an organic compound with the formula C6H5N3. It is one of the prototypical organic azides. It has a pungent odor. The structure consists of a linear azide substituent bound to a phenyl group...
to an aniline
Aniline
Aniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Being a precursor to many industrial chemicals, its main use is in the manufacture of precursors to polyurethane...
:
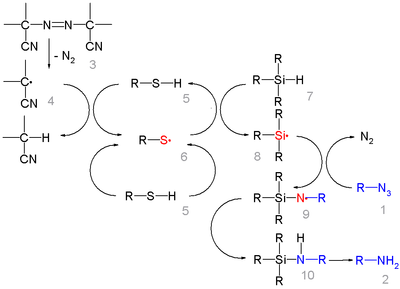
In this reaction ACCN is a radical initiator
Radical initiator
In chemistry, radical initiators are substances that can produce radical species under mild conditions and promote radical reactions . These substances generally possess weak bonds—bonds that have small bond dissociation energies. Radical initiators are utilized in industrial processes such...
and an aliphatic thiol
Thiol
In organic chemistry, a thiol is an organosulfur compound that contains a carbon-bonded sulfhydryl group...
transfers radical character to the silylhydride. The triethylsilyl free radical then reacts with the azide with expulsion of nitrogen to a N-silylarylaminyl radical which abstracts a proton from a thiol completing the catalytic cycle
Catalytic cycle
A catalytic cycle in chemistry is a term for a multistep reaction mechanism that involves a catalyst . The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, materials science, etc. Often such cycles show the conversion of a...
:
Aqueous workup then gives aniline.
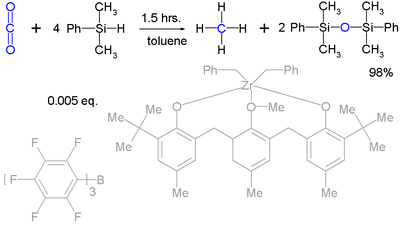
Silyl hydrides can even take up the reduction of robust molecules such as carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
(to methane
Methane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
) :
Although it takes a very complex catalyst system.
Hydrosilylation
Silyl hydrides react with various unsaturated substrates such as alkeneAlkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s, alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
s, imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
s, carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
s and oxime
Oxime
An oxime is a chemical compound belonging to the imines, with the general formula R1R2C=NOH, where R1 is an organic side chain and R2 may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds...
s to new organosilicon compounds in hydrosilylation. In the reaction of triphenylsilyl hydride with phenylacetylene
Phenylacetylene
Phenylacetylene is an alkyne hydrocarbon containing a phenyl group. It exists as a colorless, viscous liquid. In research, it is sometimes used as an analog for acetylene; being a liquid, it is easier to handle than acetylene gas.-Preparation:...
the reaction product is a trans or cis or the geminal
Geminal
In chemistry, the term geminal refers to the relationship between two functional groups that are attached to the same atom...
vinyl silane, for example :
In the related silylmetalation, a metal replaces the hydrogen atom.
Silenes
Organosilicon compounds, unlike their carbon counterparts, do not have a rich double bondDouble bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
chemistry due to the large difference in electronegativity . Existing compounds with silene Si=C bonds (also known as alkylidenesilanes) are laboratory curiosities such as the silicon benzene analogue silabenzene
Silabenzene
A silabenzene is a heteroaromatic compound containing one or more silicon atoms instead of carbon atom in benzene. A single substitution gives silabenzene proper; additional substitutions give a disilabenzene , trisilabenzene , etc.Silabenzenes have been the targets of many theoretical and...
. In 1967, Gusel'nikov and Flowers provided the first evidence for silenes from pyrolysis of dimethylsilacyclobutane . The first stable (kinetically shielded) silene was reported in 1981 by Brook

Disilene
Disilene
Disilenes are compounds containing a silicon–silicon double bond and are considered to be heavier analogues of alkenes. They are sometimes also called disilaalkenes.-History:...
s have Si=Si double bonds and disilyne
Disilyne
A disilyne is a chemical compound that contains a formal silicon - silicon triple bond and as such is formulated R2Si2 and is the silicon analogue of an alkyne. The hypothetical parent hydride, Si2H2 does not actually exist, all attempts to synthesize it results in a diradical disilene, with the...
s are silicon analogues of an alkyne. The first Silyne (with a silicon to carbon triple bond) was reported in 2010
Siloles
Siloles, also called silacyclopentadienes, are members of a larger class of compounds called metalloles, are the silicon analogs of pyrrolePyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4H4NH. It is a colourless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3...
s and are of current academic interest due to their electroluminescence
Electroluminescence
Electroluminescence is an optical phenomenon and electrical phenomenon in which a material emits light in response to the passage of an electric current or to a strong electric field...
and other electronic properties. Siloles are efficient in electron transport. They owe their low lying LUMO
Lumo
Lumo is a 2007 documentary film about twenty-year-old Lumo Sinai, a woman who fell victim to "Africa's First World War." While returning home one day, Lumo and another woman were gang-raped by a group of soldiers fighting for control of the Democratic Republic of the Congo during the 1994 Rwandan...
to a favorable interaction between the antibonding
Antibonding
Antibonding is a type of chemical bonding. An antibonding orbital is a form of molecular orbital that is located outside the region of two distinct nuclei...
sigma
Sigma bond
In chemistry, sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most clearly defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a σ-bond is...
silicon orbital with a antibonding
Antibonding
Antibonding is a type of chemical bonding. An antibonding orbital is a form of molecular orbital that is located outside the region of two distinct nuclei...
pi orbital of the butadiene fragment.
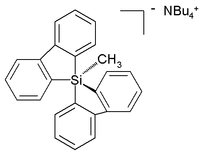
Hypercoordinated silicon
Unlike carbon, silicon compounds can be coordinated to five atoms as well in a group of compounds ranging from so-called silatranes, such as PhenylsilatranePhenylsilatrane
Phenylsilatrane is a convulsant chemical which has been used as a rodenticide....
, to a uniquely stable pentaorganosilicate :
See also
- Compounds of carbon with period 3 elementPeriod 3 elementA period 3 element is one of the chemical elements in the third row of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when chemical...
s: organoaluminum compounds, organosilicon compounds, organophosphorus compounds, organosulfur compounds, - Compounds of carbon with other group 14Carbon groupThe carbon group is a periodic table group consisting of carbon , silicon , germanium , tin , lead , and ununquadium ....
elements: organosilicon compounds, organogermanium compoundOrganogermanium compoundOrganogermanium compounds are organometallic compounds containing a carbon to germanium or hydrogen to germanium chemical bond. Organogermanium chemistry is the corresponding chemical science...
s, organotin compounds, organolead compoundOrganolead compoundOrganolead compounds are chemical compounds containing a chemical bond between carbon and lead. Organolead chemistry is the corresponding science. The first organolead was hexaethyldilead synthesised in 1858. Sharing the same group with carbon, lead is tetravalent.Going down the carbon group the...
s.
- silyleneSilyleneSilylenes are chemical compounds containing a divalent silicon atom without any electrical charge. Both dicoordinate and tricoordinate silylenes are reported in the literature. They are considered to be heavier analogues of carbene. In earlier times, they were called silene, but this is a mistake,...
s, the carbeneCarbeneIn chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
counterparts and silylenoidSilylenoidA Silylenoid in organosilicon chemistry is a type of chemical compound with the general structure R2SiXM where R is any organic residue, X a halogen and M a metal. Silylenoids are the silicon pendants of carbenoid and both compounds have carbene or silylene like properties.Silylenoids are...
s the carbenoidCarbenoidIn chemistry a carbenoid is a reactive intermediate that shares reaction characteristics with a carbene. In the Simmons-Smith reaction the carbenoid intermediate is a zinc / iodine complex that takes the form of...
counterparts.
External links
- Magnus Walter's Selected Aspects of Organosilicon Chemistry
- Silicon in organic synthesis
- Safety data for methyltrichlorosilane from the Chemistry Department at Oxford University.
- S. Marsden (Editor): Contemporary organosilicon chemistry. Thematic Series in the Open Access Beilstein Journal of Organic Chemistry.