
Allyl
Encyclopedia
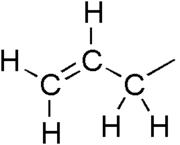
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
with the structural formula H2C=CH-CH2R, where R is the connection to the rest of the molecule. It is made up of a methylene
Methylene
Methylene is a chemical species in which a carbon atom is bonded to two hydrogen atoms. Three different possibilities present themselves:* the -CH2- substituent group: e.g., dichloromethane ....
(-CH2-), attached to a vinyl
Vinyl
A vinyl compound is any organic compound that contains a vinyl group ,which are derivatives of ethene, CH2=CH2, with one hydrogen atom replaced with some other group...
group (-CH=CH2). The name is derived from the Latin word for garlic
Garlic
Allium sativum, commonly known as garlic, is a species in the onion genus, Allium. Its close relatives include the onion, shallot, leek, chive, and rakkyo. Dating back over 6,000 years, garlic is native to central Asia, and has long been a staple in the Mediterranean region, as well as a frequent...
, Allium sativum. Theodor Wertheim
Theodor Wertheim
Theodor Wertheim was an Austrian chemist born in Vienna.He was privatdozent in Vienna, and a professor at the University of Pest from 1853 to 1860. Afterwards he returned to Vienna, and beginning in 1861 worked at the University of Graz. In May, 1864 he moved back to Vienna, where he died soon...
isolated an allyl derivative from garlic oil and named it Schwefelallyl. The term allyl applies to many compounds related to H2C=CH-CH2, which includes thousands of different chemical compounds, some of which are of practical or everyday importance.
Nomenclature and bonding
Allyl is a widely used term in organic chemistry. The unpaired electron is delocalized. Allylic radicals, anions, and cations are often discussed as intermediates in reactions. All feature three contiguous sp²-hybridized carbon centers.Allylic sites
A site on the saturated carbon atom is called the "allylic position" or "allylic site." A group attached at this site is sometimes described as "allylic." Thus, CH2=CHCH2OH "has an allylic hydroxyl group." Allylic C-H bonds are about 15% weaker than the C-H bonds in ordinary sp3 carbon centers and are thus more reactive. As one practical consequence of this heightened reactivity, the production of acrylonitrileAcrylonitrile
Acrylonitrile is the chemical compound with the formula C3H3N. This pungent-smelling colorless liquid often appears yellow due to impurities. It is an important monomer for the manufacture of useful plastics. In terms of its molecular structure, it consists of a vinyl group linked to a nitrile...
exploits the easy oxidation of the allylic C-H centers in propene. Benzylic and allylic are related in terms of structure, bond strength, and reactivity. Other reactions that tend to occur with allylic compounds are allylic oxidations, ene reaction
Ene reaction
The Ene reaction is a chemical reaction between an alkene with an allylic hydrogen and a compound containing a multiple bond , in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the...
s, and the Tsuji–Trost reaction. Benzyl
Benzyl
In organic chemistry, benzyl is the term used to describe the substituent or molecular fragment possessing the structure C6H5CH2-. Benzyl features a benzene ring attached to a CH2 group.-Nomenclature:...
ic groups are related to allyl groups; both show enhanced reactivity.
Pentadienyl
A CH2 group connected to two vinyl groups is said to be doubly allylic. The bond dissociation energyBond dissociation energy
In chemistry, bond-dissociation energy or D0, is one measure of the bond strength in a chemical bond. It is defined as the standard enthalpy change when a bond is cleaved by homolysis, with reactants and products of the homolysis reaction at 0 K...
of C-H bonds on a doubly allylic centre is about 10% less than the bond dissociation energy of a C-H bond that is allylic. The weakened C-H bonds reflect the high stability of the resulting "pentadienyl" radicals. Compounds containing the C=C-CH2-C=C linkages, e.g. linoleic acid
Linoleic acid
Linoleic acid is an unsaturated n-6 fatty acid. It is a colorless liquid at room temperature. In physiological literature, it has a lipid number of 18:2...
derivatives, are prone to autoxidation, which can lead to polymerization or form semisolids. This reactivity pattern is fundamentally related to the film-forming behavior of the "drying oil
Drying oil
A drying oil is an oil that hardens to a tough, solid film after a period of exposure to air. The oil hardens through a chemical reaction in which the components crosslink by the action of oxygen . Drying oils are a key component of oil paint and some varnishes...
s" which are components of oil paints and varnish
Varnish
Varnish is a transparent, hard, protective finish or film primarily used in wood finishing but also for other materials. Varnish is traditionally a combination of a drying oil, a resin, and a thinner or solvent. Varnish finishes are usually glossy but may be designed to produce satin or semi-gloss...
es.
Homoallylic
The term homoallylic refers to the position on a carbon skeleton next to an allylic position. In CH2=CHCH2CH2Cl, the chloride occupies a homoallylic position.Allyl compounds
Many substituents can be attached to the allyl group to give stable compounds. Allyl alcoholAllyl alcohol
Allyl alcohol is an organic compound with the structural formula CH2=CHCH2OH. Like many alcohols,it is a water soluble, colourless liquid, but it is more toxic than typical small alcohols. Allyl alcohol is used as a raw material for the production of glycerol, but is used as a precursor to many...
has the structure H2C=CH-CH2OH. It contains two sp²-hybridized carbon centers and one sp3-hybridized carbon center. Another example of a simple allyl compound is allyl chloride
Allyl chloride
Allyl chloride is the organic compound with the formula CH2=CHCH2Cl. This colorless liquid is insoluble in water but soluble in common organic solvents. It is mainly converted to epichlorohydrin, used in the production of plastics. It is a chlorinated derivative of propylene.-Production:Allyl...
. Substituted versions of the parent allyl group, such as the trans-but-2-en-1-yl or crotyl group (CH3CH=CH-CH2-), may also be referred to as allylic groups. Many allylic compounds are lachrymatory.
Allyl ligands in organometallic chemistry
The allyl ligand is commonly encountered in organometallic chemistryOrganometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
. Most commonly, allyl ligands bind to metals via all three carbon centers, the so-called η3-binding mode. η1-allyl complexes are also known. The most popular allyl reagent is probably allyl palladium chloride. They are usually generated by oxidative addition of allylic halides. This route affords pi-allyl nickel compounds, such as (allyl)2Ni2Cl2:
- 2 Ni(CO)4 + 2 ClCH2CH=CH2 → Ni2(μ-Cl)2(η3-C3H5)2 + 8 CO

