
Carbonyl
Encyclopedia
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
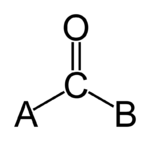
, a carbonyl group is a functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
composed of a carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
double-bonded
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
to an oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups.
The term carbonyl can also refer to carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
as a ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
in an inorganic or organometallic complex (a metal carbonyl
Metal carbonyl
Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. These complexes may be homoleptic, that is containing only CO ligands, such as nickel carbonyl , but more commonly metal carbonyls contain a mix of ligands, such as Re3Cl...
, e.g. nickel carbonyl
Nickel carbonyl
Nickel carbonyl is the organonickel compound with the formula Ni4. This pale-yellow liquid is the principal carbonyl of nickel. It is an intermediate in the Mond process for the purification of nickel and a reagent in organometallic chemistry...
).
The remainder of this article concerns itself with the organic chemistry definition of carbonyl, where carbon and oxygen share a double bond.
Carbonyl compounds
A carbonyl group characterizes the following types of compounds:| Compound | Aldehyde Aldehyde An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group.... |
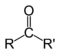
Ketone Ketone In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology... |
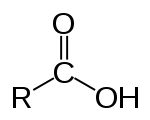
Carboxylic acid Carboxylic acid Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group... |
Ester Ester Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and... |
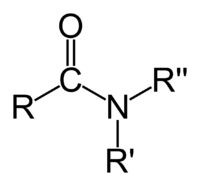
Amide Amide In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an... |
Enone Enone An enone is an unsaturated chemical compound or functional group consisting of a conjugated system of an alkene and a ketone. The simplest enone is methyl vinyl ketone or CH2=CHCOCH3.... |
Acyl halide Acyl halide An acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group.... |
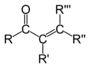
Acid anhydride |
| Structure | 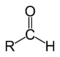 |
 |
 |
 |
 |
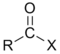 |
 |
|
| General formula | RCHO | RCOR' | RCOOH | RCOOR' | | |
RCOX | (RCO)2O | |
Note that the most specific labels are usually employed. For example, R(CO)O(CO)R' structures are known as acid anhydride rather than the more generic ester, even though the ester motif is present.
Other organic carbonyls are urea
Urea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
and the carbamate
Carbamate
Carbamates are organic compounds derived from carbamic acid . A carbamate group, carbamate ester, and carbamic acids are functional groups that are inter-related structurally and often are interconverted chemically. Carbamate esters are also called urethanes.-Synthesis:Carbamic acids are derived...
s, the derivatives of acyl chloride
Acyl chloride
In organic chemistry, an acyl chloride is an organic compound with the functional group -CO-Cl. Their formula is usually written RCOCl, where R is a side chain. They are usually considered to be reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride,...
s chloroformate
Chloroformate
Chloroformates are a class of chemical compounds which are esters of chloroformic acid. They are widely used as reagents in organic chemistry. For example, benzyl chloroformate is used to introduce the CBZ protecting group and fluorenylmethyloxycarbonylchloride is used to introduce the FMOC...
s and phosgene
Phosgene
Phosgene is the chemical compound with the formula COCl2. This colorless gas gained infamy as a chemical weapon during World War I. It is also a valued industrial reagent and building block in synthesis of pharmaceuticals and other organic compounds. In low concentrations, its odor resembles...
, carbonate ester
Carbonate ester
A carbonate ester is a functional group in organic chemistry consisting of a carbonyl group flanked by two alkoxy groups. The general structure of these carbonates is R1OOR2 and they are related to esters R1OR and ethers R1OR2 and also to the inorganic carbonates.Carbonate esters are used as...
s, thioester
Thioester
Thioesters are compounds with the functional group C-S-CO-C. They are the product of esterification between a carboxylic acid and a thiol. Thioesters are widespread in biochemistry, the best-known derivative being acetyl-CoA.-Synthesis:...
s, lactone
Lactone
In chemistry, a lactone is a cyclic ester which can be seen as the condensation product of an alcohol group -OH and a carboxylic acid group -COOH in the same molecule...
s, lactam
Lactam
A lactam is a cyclic amide. Prefixes indicate how many carbon atoms are present in the ring: β-lactam , γ-lactam , δ-lactam...
s, hydroxamates
Hydroxamic acid
A hydroxamic acid is a class of chemical compounds sharing the same functional group in which an hydroxylamine is inserted into a carboxylic acid. Its general structure is R-CO-NH-OH, with an R as an organic residue, a CO as a carbonyl group, and a hydroxylamine as NH2-OH. They are used as metal...
, and isocyanate
Isocyanate
Isocyanate is the functional group of elements –N=C=O , not to be confused with the cyanate functional group which is arranged as –O–C≡N or with isocyanide, R-N≡C. Any organic compound which contains an isocyanate group may also be referred to in brief as an isocyanate. An isocyanate may have more...
s. Examples of inorganic carbonyl compounds are carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
and carbonyl sulfide
Carbonyl sulfide
Carbonyl sulfide is the chemical compound with the formula OCS. Commonly written as COS, it is a colourless flammable gas with an unpleasant odor. It is a linear molecule consisting of a carbonyl group double bonded to a sulfur atom...
.
A special group of carbonyl compounds are 1,3-dicarbonyl compounds that have acidic protons in the central methylene unit. Examples are Meldrum's acid
Meldrum's acid
Meldrum's acid or 2,2-dimethyl-1,3-dioxane-4,6-dione is an organic compound. The compound was first made in 1908 by Andrew Norman Meldrum by a condensation reaction of malonic acid with acetone in acetic anhydride and sulfuric acid. Meldrum misidentified the structure as a β-lactone of...
, diethyl malonate
Diethyl malonate
Diethyl malonate, also known as DEM, is the diethyl ester of malonic acid. It occurs naturally in grapes and strawberries as a colourless liquid with an apple-like odour, and is used in perfumes...
and acetylacetone
Acetylacetone
Acetylacetone is an organic compound that famously exists in two tautomeric forms that rapidly interconvert. The less stable tautomer is a diketone formally named pentane-2,4-dione. The more common tautomer is the enol form. The pair of tautomers rapidly interconvert and are treated as a single...
.
Reactivity
Oxygen is more electronegative than carbon, and thus pulls electron density away from carbon to increase the bond's polarity. Therefore, the carbonyl carbon becomes electrophilicElectrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
, and thus more reactive with nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
s. Also, the electronegative oxygen can react with an electrophile; for example a proton in an acidic solution or other Lewis Acid.
The alpha hydrogens of a carbonyl compound are much more acidic (~103 times more acidic) than a typical C-H bond. For example, the pKa values of acetaldehyde
Acetaldehyde
Acetaldehyde is an organic chemical compound with the formula CH3CHO or MeCHO. It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale industrially. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants as part...
and acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
are 16.7 and 19, respectively. This is because a carbonyl is in tautomeric resonance
Resonance (chemistry)
In chemistry, resonance or mesomerism is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis formula...
with an enol
Enol
Enols are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated anions of enols are called enolates...
. The deprotonation of the enol with a strong base produces an enolate, which is a powerful nucleophile and can alkylate electrophiles such as other carbonyls.
Amides are the most stable of the carbonyl couplings due to their high resonance stabilization between the nitrogen-carbon and carbon-oxygen bonds.
Carbonyl groups can be reduced
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
by reaction with hydride
Hydride
In chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties. In compounds that are regarded as hydrides, hydrogen is bonded to a more electropositive element or group...
reagents such as NaBH4
Sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate, is an inorganic compound with the formula NaBH4. This white solid, usually encountered as a powder, is a versatile reducing agent that finds wide application in chemistry, both in the laboratory and on a technical scale. Large amounts are...
and LiAlH4
Lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
, or catalytically by hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
and a catalyst such as copper chromite, Raney nickel
Raney nickel
Raney nickel is a solid catalyst composed of fine grains of a nickel-aluminium alloy, used in many industrial processes. It was developed in 1926 by American]] engineer Murray Raney as an alternative catalyst for the hydrogenation of vegetable oils in industrial processes...
, rhenium
Rhenium
Rhenium is a chemical element with the symbol Re and atomic number 75. It is a silvery-white, heavy, third-row transition metal in group 7 of the periodic table. With an average concentration of 1 part per billion , rhenium is one of the rarest elements in the Earth's crust. The free element has...
, ruthenium
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
or even rhodium
Rhodium
Rhodium is a chemical element that is a rare, silvery-white, hard and chemically inert transition metal and a member of the platinum group. It has the chemical symbol Rh and atomic number 45. It is composed of only one isotope, 103Rh. Naturally occurring rhodium is found as the free metal, alloyed...
. Ketones give secondary alcohols; aldehydes, esters and carboxylic acids give primary alcohols.
Carbonyls would be alkylated
Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion or a carbene . Alkylating agents are widely used in chemistry because the alkyl group is probably the most common group encountered in...
by nucleophilic attack by organometallic reagents such as organolithium reagents and Grignard reagents. Carbonyls also may be alkylated by enolates as in aldol reaction
Aldol reaction
The aldol reaction is a powerful means of forming carbon–carbon bonds in organic chemistry.Discovered independently by Charles-Adolphe Wurtz and Alexander Porfyrevich Borodin in 1872, the reaction combines two carbonyl compounds to form a new β-hydroxy carbonyl compound...
s. Carbonyls are also the prototypical groups with vinylogous
Vinylogous
Vinylogous is an adjective used to apply the concept of vinylogy taught in intermediate undergraduate through graduate/research organic chemistry. Vinylogy has been defined as the transmission of electronic effects through a conjugated organic bonding system...
reactivity, e.g. the Michael reaction
Michael reaction
The Michael reaction or Michael addition is the nucleophilic addition of a carbanion or another nucleophile to an alpha, beta unsaturated carbonyl compound. It belongs to the larger class of conjugate additions. This is one of the most useful methods for the mild formation of C-C bonds...
where an unsaturated carbon in conjugation with the carbonyl is alkylated instead of the carbonyl itself.
Other important reactions include:
- Wittig ReactionWittig reactionThe Wittig reaction is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide to give an alkene and triphenylphosphine oxide....
a phosphonium ylid is used to create an alkene - Wolff-Kishner reductionWolff-Kishner reductionThe Wolff–Kishner reduction is a chemical reaction that fully reduces a ketone to an alkane.The method originally involved heating the hydrazine with sodium ethoxide in a sealed vessel at about 180 °C. Other bases have been found equally effective...
into a hydrazoneHydrazoneHydrazones are a class of organic compounds with the structure R1R2C=NNH2. They are related to ketones and aldehydes by the replacement of the oxygen with the NNH2 functional group...
and further into a saturated alkane - Clemmensen reductionClemmensen reductionClemmensen reduction is a chemical reaction described as a reduction of ketones to alkanes using zinc amalgam and hydrochloric acid. This reaction is named after Erik Christian Clemmensen, a Danish chemist....
into a saturated alkane - Conversion into thioacetalThioacetalThioacetals are the sulfur analogue of acetals. They are prepared in a similar way to acetals: by reacting a thiol with an aldehyde:Dithioacetals are prepared similarly to thioacetals, which are intermediates:...
s - HydrationHydration reactionIn organic chemistry, a hydration reaction is a chemical reaction in which a hydroxyl group and a hydrogen cation are added to the two carbon atoms bonded together in the carbon-carbon double bond which makes up an alkene functional group. The reaction usually runs in a strong acidic, aqueous...
to hemiacetalHemiacetalHemiacetals and hemiketals are compounds that are derived from aldehydes and ketones respectively. The Greek word hèmi means half...
s and hemiketals, and then to acetalAcetalAn acetal is a molecule with two single-bonded oxygen atoms attached to the same carbon atom.Traditional usages distinguish ketals from acetals...
s and ketals - Reaction with ammoniaAmmoniaAmmonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
and primary amineAmineAmines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s to form imineImineAn imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
s - Reaction with hydroxylamineHydroxylamineHydroxylamine is an inorganic compound with the formula NH2OH. The pure material is a white, unstable crystalline, hygroscopic compound. However, hydroxylamine is almost always provided and used as an aqueous solution. It is used to prepare oximes, an important functional group. It is also an...
s to form oximeOximeAn oxime is a chemical compound belonging to the imines, with the general formula R1R2C=NOH, where R1 is an organic side chain and R2 may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds...
s - Reaction with cyanideCyanideA cyanide is a chemical compound that contains the cyano group, -C≡N, which consists of a carbon atom triple-bonded to a nitrogen atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic....
anion to form cyanohydrinCyanohydrinA cyanohydrin is a functional group found in organic compounds. Cyanohydrins have the formula R2CCN, where R is H, alkyl, or aryl. Cyanohydrins are industrially important precursors to carboxylic acids and some amino acids...
s - Oxidation with oxaziridineOxaziridineAn oxaziridine is an organic molecule that features a three-membered heterocycle containing oxygen, nitrogen, and carbon.-History:Oxaziridine derivatives were first synthesized in the mid 1950s by Emmons and subsequently by Krimm and Horner and Jürgens...
s to acyloins - Reaction with Tebbe's reagentTebbe's reagentThe Tebbe reagent is the organometallic compound with the formula 2TiCH2ClAl2. It used in the methylenation of carbonyl compounds, that is it converts organic compounds containing the R2C=O group into the related R2C=CH2 derivative...
and phosphonium ylides to alkeneAlkeneIn organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s. - Perkin reactionPerkin reactionThe Perkin reaction is an organic reaction developed by William Henry Perkin that can be used to make cinnamic acids i.e. α-β-unsaturated aromatic acid by the aldol condensation of aromatic aldehydes and acid anhydrides in the presence of an alkali salt of the acid.Several reviews have been written....
, an aldol reactionAldol reactionThe aldol reaction is a powerful means of forming carbon–carbon bonds in organic chemistry.Discovered independently by Charles-Adolphe Wurtz and Alexander Porfyrevich Borodin in 1872, the reaction combines two carbonyl compounds to form a new β-hydroxy carbonyl compound...
variant - Aldol condensationAldol condensationAn aldol condensation is an organic reaction in which an enol or an enolate ion reacts with a carbonyl compound to form a β-hydroxyaldehyde or β-hydroxyketone, followed by a dehydration to give a conjugated enone....
, a reaction between an enolate and a carbonyl - Cannizzaro reactionCannizzaro reactionThe Cannizzaro reaction, named after its discoverer Stanislao Cannizzaro, is a chemical reaction that involves the base-induced disproportionation of an aldehyde lacking a hydrogen atom in the alpha position...
, a disproportionation of aldehydes into alcohols and acids - Tishchenko reactionTishchenko reactionThe Tishchenko reaction is a chemical reaction that involves disproportionation of an aldehyde lacking a hydrogen atom in the alpha position in the presence of an alkoxide. The reaction product is an ester. Catalysts are aluminium alkoxides or sodium alkoxides...
, another disproportionation of aldehydes that gives a dimeric ester
α,β-Unsaturated carbonyl compounds
α,β-Unsaturated carbonyl compounds are an important class of carbonyl compounds with the general structure Cβ=Cα−(C=O)−. In these compounds the carbonyl group is conjugatedConjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
with an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
(hence the adjective
Adjective
In grammar, an adjective is a 'describing' word; the main syntactic role of which is to qualify a noun or noun phrase, giving more information about the object signified....
unsaturated
Saturation (chemistry)
In chemistry, saturation has six different meanings, all based on reaching a maximum capacity...
), from which they derive special properties. Unlike simple carbonyls, they exhibit vinylogous
Vinylogous
Vinylogous is an adjective used to apply the concept of vinylogy taught in intermediate undergraduate through graduate/research organic chemistry. Vinylogy has been defined as the transmission of electronic effects through a conjugated organic bonding system...
reactivity and can be used to form carbon-carbon bonds (as in alkylation
Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion or a carbene . Alkylating agents are widely used in chemistry because the alkyl group is probably the most common group encountered in...
). Examples of unsaturated carbonyls are acrolein
Acrolein
Acrolein is the simplest unsaturated aldehyde. It is produced widely but is most often immediately reacted with other products due to its instability and toxicity...
(propenal), mesityl oxide
Mesityl oxide
Mesityl oxide is a α,β-Unsaturated ketone with the formula CH3CCH=C2. This compound is a colorless, volatile liquid with a strong peppermint odor.-Synthesis:...
, acrylic acid
Acrylic acid
Acrylic acid is an organic compound with the formula CH2=CHCO2H. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a characteristic acrid or tart smell. It is miscible with water, alcohols,...
, and maleic acid
Maleic acid
Maleic acid is an organic compound that is a dicarboxylic acid, a molecule with two carboxyl groups. Maleic acid is the cis-isomer of butenedioic acid, whereas fumaric acid is the trans-isomer...
. Unsaturated carbonyls can be prepared in the laboratory in an aldol reaction
Aldol reaction
The aldol reaction is a powerful means of forming carbon–carbon bonds in organic chemistry.Discovered independently by Charles-Adolphe Wurtz and Alexander Porfyrevich Borodin in 1872, the reaction combines two carbonyl compounds to form a new β-hydroxy carbonyl compound...
and in the Perkin reaction
Perkin reaction
The Perkin reaction is an organic reaction developed by William Henry Perkin that can be used to make cinnamic acids i.e. α-β-unsaturated aromatic acid by the aldol condensation of aromatic aldehydes and acid anhydrides in the presence of an alkali salt of the acid.Several reviews have been written....
.
The carbonyl group draws electrons away from the alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
, and the alkene group is, therefore, deactivated towards an electrophile
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
, such as bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
or hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
.
As a general rule with unsymmetric electrophiles, hydrogen attaches itself at the α-position in an electrophilic addition
Electrophilic addition
In organic chemistry, an electrophilic addition reaction is an addition reaction where, in a chemical compound, a π bond is broken and two new σ bonds are formed...
. On the other hand, these compounds are activated towards nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
s in nucleophilic conjugate addition
Nucleophilic conjugate addition
Nucleophilic conjugate addition is a type of organic reaction. Ordinary nucleophilic additions or 1,2-nucleophilic additions deal mostly with additions to carbonyl compounds. Simple alkene compounds do not show 1,2 reactivity due to lack of polarity, unless the alkene is activated with special...
.
Since α,β-unsaturated compounds are electrophiles, many α,β-unsaturated carbonyl compounds are toxic, mutagenic and carcinogenic. DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
can attack the β carbon and thus be alkylated
Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion or a carbene . Alkylating agents are widely used in chemistry because the alkyl group is probably the most common group encountered in...
. However, the endogenous scavenger compound glutathione
Glutathione
Glutathione is a tripeptide that contains an unusual peptide linkage between the amine group of cysteine and the carboxyl group of the glutamate side-chain...
naturally protects from toxic electrophiles in the body.
Spectroscopy
- Infrared spectroscopyInfrared spectroscopyInfrared spectroscopy is the spectroscopy that deals with the infrared region of the electromagnetic spectrum, that is light with a longer wavelength and lower frequency than visible light. It covers a range of techniques, mostly based on absorption spectroscopy. As with all spectroscopic...
: the C=O double bond absorbs infraredInfraredInfrared light is electromagnetic radiation with a wavelength longer than that of visible light, measured from the nominal edge of visible red light at 0.74 micrometres , and extending conventionally to 300 µm...
light at wavenumberWavenumberIn the physical sciences, the wavenumber is a property of a wave, its spatial frequency, that is proportional to the reciprocal of the wavelength. It is also the magnitude of the wave vector...
s between approximately 1600–1900 cm−1. The exact location of the absorption is well understood with respect to the geometry of the molecule. This absorption is known as the "carbonyl stretch" when displayed on an infrared absorption spectrum. - Nuclear magnetic resonanceNuclear magnetic resonanceNuclear magnetic resonance is a physical phenomenon in which magnetic nuclei in a magnetic field absorb and re-emit electromagnetic radiation...
: the C=O double-bond exhibits different resonances depending on surrounding atoms, generally a downfield shift. The 13C NMR of a carbonyl carbon is in the range of 160-220 ppm.
Further reading
- L.G. Wade, Jr. Organic Chemistry, 5th ed. Prentice HallPrentice HallPrentice Hall is a major educational publisher. It is an imprint of Pearson Education, Inc., based in Upper Saddle River, New Jersey, USA. Prentice Hall publishes print and digital content for the 6-12 and higher-education market. Prentice Hall distributes its technical titles through the Safari...
, 2002. ISBN 0-13-033832-X - The Frostburg State UniversityFrostburg State UniversityFrostburg State University is a four-year university located on a campus in Frostburg, Maryland, in Western Maryland, and is part of the University System of Maryland. FSU is accredited by the Middle States Commission on Higher Education.-History:...
Chemistry Department. Organic Chemistry Help (2000). - Advanced Chemistry Development, Inc. IUPAC Nomenclature of Organic Chemistry (1997).
- William Reusch. tara VirtualText of Organic Chemistry (2004).
- Purdue Chemistry Department http://chemed.chem.purdue.edu/genchem/topicreview/bp/2organic/carbonyl.html (retrieved Sep 2006). Includes water solubility data.
- William Reusch. (2004) Aldehydes and Ketones Retrieved 23 May 2005.
- ILPI. (2005) The MSDS Hyperglossary- Anhydride.

