Beta-silicon effect
Encyclopedia
The beta-silicon effect also called silicon hyperconjugation in organosilicon chemistry
is a special type of hyperconjugation
and describes the stabilizing effect of a silicon
atom placed in a position one removed (β) from a carbocation
. A prerequisite is an antiperiplanar relationship between the two groups. Silicon hyperconjugation explains specific observations regarding chemical kinetics
and stereochemistry
of organic reactions with reactants containing silicon.
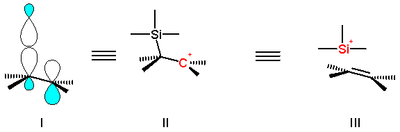
The effect is understood in terms of classical hyperconjugation depicted in structure 3 in scheme 1 or in terms of molecular orbital
overlap 1 which is a stabilizing overlap between the empty p-orbital of the carbocation and the filled sigma molecular orbital of the silicon to carbon bond.
 The alpha-silicon effect is the destabilizing effect of a silicon atom next to a reaction center with a partial positive charge.
The alpha-silicon effect is the destabilizing effect of a silicon atom next to a reaction center with a partial positive charge.
In a pioneering study by Frank C. Whitmore
ethyltrichlorosilane (scheme 2) was chlorinated
by sulfuryl chloride
as chlorine donor and benzoyl peroxide
as radical initiator
in a radical substitution
resulting in chloride monosubstitution to some extent in the α-position (28%, due to steric hindrance of the silyl group) and predominantly in the β-position.
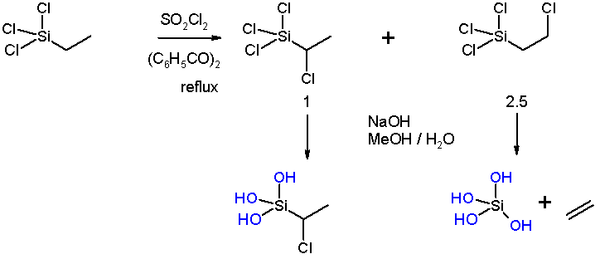 By adding sodium hydroxide to the α-substituted compound only the silicon chlorine groups are replaced but not the carbon chlorine group. Addition of alkali to the β-substituted compound on the other hand leads to an elimination reaction
By adding sodium hydroxide to the α-substituted compound only the silicon chlorine groups are replaced but not the carbon chlorine group. Addition of alkali to the β-substituted compound on the other hand leads to an elimination reaction
with liberation of ethylene
.
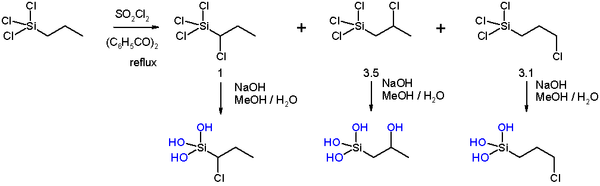
In another set of experiments (scheme 3) the chlorination is repeated with n-propyltrichlorosilane The α-adduct and the γ-adduct are resistant to hydrolysis but the chlorine group in the β-adduct gets replaced by a hydroxyl
group.
 The silicon effect is also manifest in certain compound properties. Trimethylsilylmethylamine (Me3SiCH2NH2) is a stronger base
The silicon effect is also manifest in certain compound properties. Trimethylsilylmethylamine (Me3SiCH2NH2) is a stronger base
with a pKa
of 10.96 for the conjugate acid
than the carbon analogue neopentyl amine with pKa 10.21. In the same vein trimethylsilylacetic acid (pKa 5.22) is a poorer acid than trimethyl acetic acid (pKa 5.00).
Organosilicon
Organosilicon compounds are organic compounds containing carbon silicon bonds. Organosilicon chemistry is the corresponding science exploring their properties and reactivity.Like carbon, the organically bound silicon is tetravalent and tetrahedral...
is a special type of hyperconjugation
Hyperconjugation
In organic chemistry, hyperconjugation is the interaction of the electrons in a sigma bond with an adjacent empty non-bonding p-orbital or antibonding π orbital or filled π orbital, to give an extended molecular orbital that increases the stability of the system...
and describes the stabilizing effect of a silicon
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
atom placed in a position one removed (β) from a carbocation
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
. A prerequisite is an antiperiplanar relationship between the two groups. Silicon hyperconjugation explains specific observations regarding chemical kinetics
Chemical kinetics
Chemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
and stereochemistry
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
of organic reactions with reactants containing silicon.
The effect is understood in terms of classical hyperconjugation depicted in structure 3 in scheme 1 or in terms of molecular orbital
Molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first...
overlap 1 which is a stabilizing overlap between the empty p-orbital of the carbocation and the filled sigma molecular orbital of the silicon to carbon bond.
In a pioneering study by Frank C. Whitmore
Frank C. Whitmore
Frank Clifford Whitmore , nicknamed "Rocky", was a prominent chemist who submitted significant evidence for the existence of carbocation mechanisms in organic chemistry.He was born in 1887 in the town of North Attleborough, Massachusetts....
ethyltrichlorosilane (scheme 2) was chlorinated
Halogenation
Halogenation is a chemical reaction that incorporates a halogen atom into a molecule in substitution of hydrogen atom. Halogenation takes place in the gas phase. There are four types of halogenation: fluorination, chlorination, bromination, and iodination...
by sulfuryl chloride
Sulfuryl chloride
Sulfuryl chloride is an inorganic compound with the formula SO2Cl2. At room temperature, it is a colorless liquid with a pungent odor. Sulfuryl chloride is not found in nature, as can be inferred from its rapid hydrolysis....
as chlorine donor and benzoyl peroxide
Benzoyl peroxide
Benzoyl peroxide is an organic compound in the peroxide family. It consists of two benzoyl groups bridged by a peroxide link. Its structural formula is [C6H5C]2O2. It is one of the most important organic peroxides in terms of applications and the scale of its production...
as radical initiator
Radical initiator
In chemistry, radical initiators are substances that can produce radical species under mild conditions and promote radical reactions . These substances generally possess weak bonds—bonds that have small bond dissociation energies. Radical initiators are utilized in industrial processes such...
in a radical substitution
Radical substitution
In organic chemistry, a radical substitution reaction is a substitution reaction involving free radicals as a reactive intermediate.The reaction always involves at least two steps, and possibly a third....
resulting in chloride monosubstitution to some extent in the α-position (28%, due to steric hindrance of the silyl group) and predominantly in the β-position.
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
with liberation of ethylene
Ethylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
.
In another set of experiments (scheme 3) the chlorination is repeated with n-propyltrichlorosilane The α-adduct and the γ-adduct are resistant to hydrolysis but the chlorine group in the β-adduct gets replaced by a hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
group.
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
with a pKa
PKA
PKA, pKa, or other similar variations may stand for:* pKa, the symbol for the acid dissociation constant at logarithmic scale* Protein kinase A, a class of cAMP-dependent enzymes* Pi Kappa Alpha, the North-American social fraternity...
of 10.96 for the conjugate acid
Conjugate acid
Within the Brønsted–Lowry acid-base theory , a conjugate acid is the acid member, HX, of a pair of two compounds that transform into each other by gain or loss of a proton. A conjugate acid can also be seen as the chemical substance that releases, or donates, a proton in the forward chemical...
than the carbon analogue neopentyl amine with pKa 10.21. In the same vein trimethylsilylacetic acid (pKa 5.22) is a poorer acid than trimethyl acetic acid (pKa 5.00).

