Hiyama coupling
Encyclopedia
In organic chemistry
, a Hiyama coupling is a palladium
or nickel
-catalyzed cross coupling reaction of organosilane
s with organic
halides or triflate
s. Hiyama couplings were first reported by Yasuo Hatanaka and Tamejiro Hiyama in 1988.
Typically, the Hiyama coupling reaction is promoted by activation of the organosilane with fluoride
s. The reaction possesses many advantages such as low environmental impact, high atom efficiency, and safe handling compared with the coupling reactions of organoboron
, organozinc, or organotin
compounds.
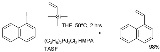
In the original 1988 publication 1-iodonaphthalene was reacted with trimethylvinylsilane to produce 1-vinylnaphthalene with allylpalladium chloride dimer
as catalyst, HMPA as solvent and TASF reagent
as fluorine source.
The reaction proceeds through the usual oxidative addition
, transmetalation
, trans-cis isomerization and reductive elimination sequence. The purpose of the fluoride is to activate the silicon compound RSiR'3 to a RSi-R'3F intermediate which is more amenable to transmetalation. Without the added fluorine the organosilicon compound is simply too stable.
In one extension the alkenylsilane is prepared in situ
from a terminal alkyne
and tetramethyldisiloxane or hexamethylcyclotrisiloxane.
In another extension the reaction is assisted by nickel chloride, LiHMDS and norephedrine with caesium fluoride
as fluorine donor:
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
, a Hiyama coupling is a palladium
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
or nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
-catalyzed cross coupling reaction of organosilane
Organosilicon
Organosilicon compounds are organic compounds containing carbon silicon bonds. Organosilicon chemistry is the corresponding science exploring their properties and reactivity.Like carbon, the organically bound silicon is tetravalent and tetrahedral...
s with organic
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
halides or triflate
Triflate
Trifluoromethanesulfonate, also known by the trivial name triflate, is a functional group with the formula CF3SO3-. The triflate group is often represented by -OTf, as opposed to -Tf...
s. Hiyama couplings were first reported by Yasuo Hatanaka and Tamejiro Hiyama in 1988.
Typically, the Hiyama coupling reaction is promoted by activation of the organosilane with fluoride
Fluoride
Fluoride is the anion F−, the reduced form of fluorine when as an ion and when bonded to another element. Both organofluorine compounds and inorganic fluorine containing compounds are called fluorides. Fluoride, like other halides, is a monovalent ion . Its compounds often have properties that are...
s. The reaction possesses many advantages such as low environmental impact, high atom efficiency, and safe handling compared with the coupling reactions of organoboron
Organoborane
Organoborane or organoboron compounds are chemical compounds that are organic derivatives of BH3, for example trialkyl boranes. Organoboron chemistry or organoborane chemistry is the chemistry of these compounds...
, organozinc, or organotin
Organotin
Organotin compounds or stannanes are chemical compounds based on tin with hydrocarbon substituents. Organotin chemistry is part of the wider field of organometallic chemistry. The first organotin compound was diethyltin diiodide, discovered by Edward Frankland in 1849...
compounds.
In the original 1988 publication 1-iodonaphthalene was reacted with trimethylvinylsilane to produce 1-vinylnaphthalene with allylpalladium chloride dimer
Allylpalladium chloride dimer
Allylpalladium chloride dimer is a chemical compound with the formula 2Pd2Cl2. This yellow air-stable compound is an important catalyst used in organic synthesis.-Synthesis and reactions:...
as catalyst, HMPA as solvent and TASF reagent
TASF reagent
The TASF reagent or trissulfonium difluorotrimethylsilicate is a reagent in organic chemistry with structural formula [3S]+[F2Si3]-. It is an anhydrous source of fluoride and is used to cleave silyl ether protective groups. Many other fluoride reagents are known, but few are truly anhydrous,...
as fluorine source.
The reaction proceeds through the usual oxidative addition
Oxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre...
, transmetalation
Transmetalation
Transmetalation is a general chemical reaction type in organometallic chemistry describing the exchange of ligands between two metal centers....
, trans-cis isomerization and reductive elimination sequence. The purpose of the fluoride is to activate the silicon compound RSiR'3 to a RSi-R'3F intermediate which is more amenable to transmetalation. Without the added fluorine the organosilicon compound is simply too stable.
In one extension the alkenylsilane is prepared in situ
In situ
In situ is a Latin phrase which translated literally as 'In position'. It is used in many different contexts.-Aerospace:In the aerospace industry, equipment on board aircraft must be tested in situ, or in place, to confirm everything functions properly as a system. Individually, each piece may...
from a terminal alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
and tetramethyldisiloxane or hexamethylcyclotrisiloxane.
In another extension the reaction is assisted by nickel chloride, LiHMDS and norephedrine with caesium fluoride
Caesium fluoride
Caesium fluoride , is an inorganic compound usually encountered as a hygroscopic white solid. It is more soluble and more readily dissociated than sodium fluoride or potassium fluoride. It is available in anhydrous form, and if water has been absorbed it is easy to dry by heating at 100 °C for...
as fluorine donor:
See also
- Coupling reactionCoupling reactionA coupling reaction in organic chemistry is a catch-all term for a variety of reactions where two hydrocarbon fragments are coupled with the aid of a metal catalyst...
- Palladium-catalyzed coupling reactionsPalladium-catalyzed coupling reactionsPalladium compounds are used as a catalyst in many coupling reactions, usually as a homogeneous catalyst. Examples include:* Heck reaction between alkenes and aryl halides* Suzuki reaction between aryl halides and boronic acids...
- Organometallic chemistryOrganometallic chemistryOrganometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...



