Peterson olefination
Encyclopedia
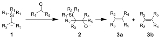
The Peterson olefination (also called the Peterson reaction) is the chemical reaction
of α-silyl carbanions 1 with ketone
s (or aldehyde
s) to form a β-hydroxysilane 2 which eliminates to form alkene
s 3.
 Several reviews have been published.
Several reviews have been published.
intermediate 3 is postulated, but no proof exists to date.
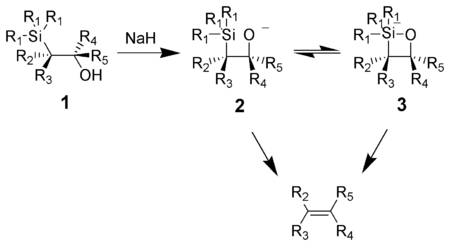 Potassium
Potassium
alkoxides eliminate quickly, while sodium
alkoxides generally require heating. Magnesium
alkoxides only eliminate in extreme conditions. The order of reactivity of alkoxides, K > Na >> Mg, is consistent with higher electron density on oxygen
, hence increasing the alkoxide nucleophilicity.
, or electron-donating substituents, the stereochemical outcome of the Peterson olefination can be controlled, because at low temperature the elimination is slow and the intermediate β-hydroxysilane can be isolated.
Once isolated, the diastereomeric β-hydroxysilanes are separated. One diastereomer
is treated with acid, while the other is treated with base, thus converted the material to an alkene with the required stereochemistry.
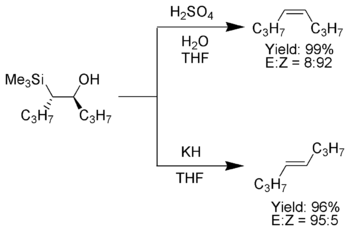
or potassium hydride
may not be feasible due to incompatible functional group
s. Chan et al. have found that acylation of the intermediate silylcarbinol with either acetyl chloride
or thionyl chloride
gives a β-silyl ester
that will eliminate spontaneously at 25°C giving the desired alkene. Corey and co-workers developed a method (sometimes dubbed the Corey-Peterson olefination) using a silylated imine to yield an α,β-unsaturated aldehyde from a carbonyl compound in one step.
For an example for its use in total synthesis see: Kuwajima Taxol total synthesis
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
of α-silyl carbanions 1 with ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s (or aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s) to form a β-hydroxysilane 2 which eliminates to form alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s 3.

Reaction mechanism
One attractive feature of the Peterson olefination is that it can be used to prepare either cis- or trans-alkenes from the same β-hydroxysilane. Treatment of the β-hydroxysilane with acid will yield one alkene, while treatment of the same β-hydroxysilane with base will yield the alkene of opposite stereochemistry.Basic elimination
The action of base upon a β-hydroxysilane 1 results in a concerted syn elimination of 2 or 3 to form the desired alkene. The penta-coordinate siliconSilicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
intermediate 3 is postulated, but no proof exists to date.

Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
alkoxides eliminate quickly, while sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
alkoxides generally require heating. Magnesium
Magnesium
Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole...
alkoxides only eliminate in extreme conditions. The order of reactivity of alkoxides, K > Na >> Mg, is consistent with higher electron density on oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
, hence increasing the alkoxide nucleophilicity.
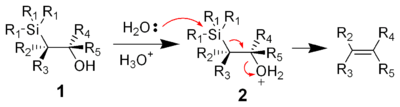
Acidic elimination
The treatment of the β-hydroxysilane 1 with acid results in protonation and an anti elimination to form the desired alkene.
Alkyl substituents
When the α-silyl carbanion contains only alkyl, hydrogenHydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
, or electron-donating substituents, the stereochemical outcome of the Peterson olefination can be controlled, because at low temperature the elimination is slow and the intermediate β-hydroxysilane can be isolated.
Once isolated, the diastereomeric β-hydroxysilanes are separated. One diastereomer
Diastereomer
Diastereomers are stereoisomers that are not enantiomers.Diastereomerism occurs when two or more stereoisomers of a compound have different configurations at one or more of the equivalent stereocenters and are not mirror images of each other.When two diastereoisomers differ from each other at...
is treated with acid, while the other is treated with base, thus converted the material to an alkene with the required stereochemistry.

Electron-withdrawing substituents
When the α-silyl carbanion contains electron-withdrawing substituents, the Peterson olefination directly forms the alkene. The intermediate β-hydroxysilane cannot be isolated as it eliminates in-situ. The basic elimination pathway has been postulated in these cases.Variations
Acidic elimination conditions are sometimes not feasible as the acid also promotes double bond isomerization. Additionally, elimation using sodiumSodium hydride
Sodium hydride is the chemical compound with the empirical formula NaH. It is primarily used as a strong base in organic synthesis. NaH is representative of the saline hydrides, meaning it is a salt-like hydride, composed of Na+ and H− ions, in contrast to the more molecular hydrides such as...
or potassium hydride
Potassium hydride
Potassium hydride, KH, is a chemical compound of potassium and hydrogen. It is a hydride of potassium. It reacts with water according to the reaction:...
may not be feasible due to incompatible functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
s. Chan et al. have found that acylation of the intermediate silylcarbinol with either acetyl chloride
Acetyl chloride
Acetyl chloride, CH3COCl, also known as ethanoyl chloride or acyl chloride, is an acid chloride derived from acetic acid. It belongs to the class of organic compounds called acyl halides. It is a colorless liquid. Acetyl chloride does not exist in nature, because contact with water would hydrolyze...
or thionyl chloride
Thionyl chloride
Thionyl chloride is an inorganic compound with the formula SOCl2. It is a reactive chemical reagent used in chlorination reactions. It is a colorless, distillable liquid at room temperature and pressure that decomposes above 140 °C. Thionyl chloride is sometimes confused with sulfuryl...
gives a β-silyl ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
that will eliminate spontaneously at 25°C giving the desired alkene. Corey and co-workers developed a method (sometimes dubbed the Corey-Peterson olefination) using a silylated imine to yield an α,β-unsaturated aldehyde from a carbonyl compound in one step.
For an example for its use in total synthesis see: Kuwajima Taxol total synthesis
Kuwajima Taxol total synthesis
The Kuwajima Taxol total synthesis by the group of Isao Kuwajima of the Tokyo Institute of Technology is one of several efforts in taxol total synthesis published in the 1990s...

