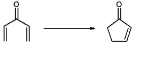
Nazarov cyclization reaction
Encyclopedia
The Nazarov cyclization reaction (often referred to as simply the Nazarov cyclization) is a chemical reaction
used in organic chemistry
for the synthesis of cyclopentenone
s. The reaction is typically divided into classical and modern variants, depending on the reagent
s and substrate
s employed. It was originally discovered by Ivan Nikolaevich Nazarov (1906–1957) in 1949 while studying the rearrangements of allyl vinyl ketones.
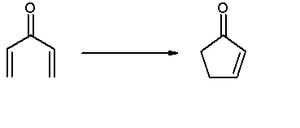 As originally described, the Nazarov cyclization involves the activation of a divinyl ketone
As originally described, the Nazarov cyclization involves the activation of a divinyl ketone
using a stoichiometric Lewis acid
or protic acid promoter. The key step of the reaction mechanism involves a cationic 4π-electrocyclic ring closure
which forms the cyclopentenone product (See Mechanism below). As the reaction has been developed, variants involving substrates other than divinyl ketones and promoters other than Lewis acids have been subsumed under the name Nazarov cyclization provided that they follow a similar mechanistic pathway
.
The success of the Nazarov cyclization as a tool in organic synthesis stems from the utility and ubiquity of cyclopentenone
s as both motifs in natural product
s (including jasmone
, the aflatoxin
s, and a subclass of prostaglandin
s) and as useful synthetic intermediates for total synthesis
. The reaction has been used in several total syntheses and several reviews have been published.
electrocyclization and is outlined below. Activation of the ketone
by the acid catalyst generates a pentadienyl cation which undergoes a thermally allowed 4π conrotatory electrocyclization as dictated by the Woodward-Hoffman rules. This generates an oxyallyl cation which undergoes an elimination reaction
to lose a β-hydrogen. Subsequent tautomerization of the enolate produces the cyclopentenone product.
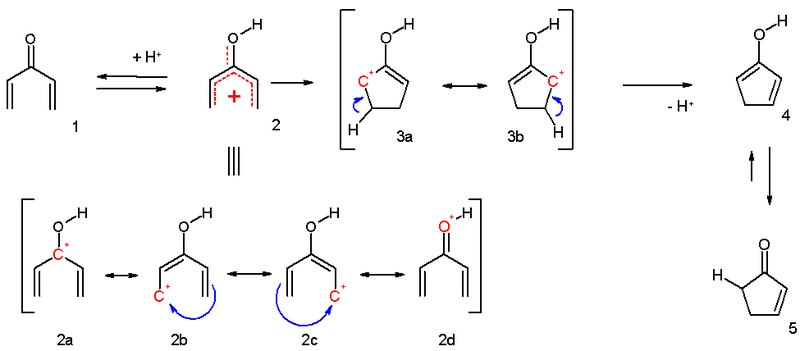 As noted above, variants that deviate from this template are known; what designates a Nazarov cyclization in particular is the generation of the pentadienyl cation followed by electrocyclic ring closure to an oxyallyl cation. In order to achieve this transformation, the molecule must be in the s-trans/s-trans conformation, placing the vinyl
As noted above, variants that deviate from this template are known; what designates a Nazarov cyclization in particular is the generation of the pentadienyl cation followed by electrocyclic ring closure to an oxyallyl cation. In order to achieve this transformation, the molecule must be in the s-trans/s-trans conformation, placing the vinyl
groups in an appropriate orientation. The propensity of the system to enter this conformation dramatically influences reaction rate
, with α-substituted substrates having an increased population of the requisite conformer due to allylic strain
. Coordination of an electron donating α-substituent by the catalyst can likewise increase the reaction rate by enforcing this conformation.
Similarly, β-substitution directed inward restricts the s-trans conformation so severely that E-Z
isomerization has been shown to occur in advance of cyclization on a wide range of substrates, yielding the trans cyclopentenone regardless of initial configuration. In this way, the Nazarov cyclization is a rare example of a stereoselective pericyclic reaction
, whereas most electrocyclizations are stereospecific. The example below uses triethylsilyl hydride
to trap the oxyallyl cation so that no elimination occurs. (See Interrupted cyclizations below)
Along this same vein, allenyl vinyl ketones of the type studied extensively by Marcus Tius of the University of Hawaii
show dramatic rate acceleration due to the removal of β-hydrogens, obviating a large amount of steric strain in the s-cis conformer.
s that marked the first major examination of this process. Nazarov correctly reasoned that the allylic olefin isomerized in situ to form a divinyl ketone before ring closure to the cyclopentenone product. The reaction shown below involves an alkyne
oxymercuration reaction
to generate the requisite ketone.
Research involving the reaction was relatively quiet in subsequent years, until in the mid-1980s when several syntheses employing the Nazarov cyclization were published. Shown below are key steps in the syntheses of Trichodiene and Nor-Sterepolide, the latter of which is thought to proceed via an unusual alkyne
-allene
isomerization that generates the divinyl ketone.
tolerance, directing the regioselectivity
of the elimination step, and improving the overall stereoselectivity
. These have been successful to varying degrees.
Additionally, modifications focused on altering the progress of the reaction, either by generating the pentadienyl cation in an unorthodox fashion or by having the oxyallyl cation "intercepted" in various ways. Furthermore, enantioselective variants of various kinds have been developed. The sheer volume of literature on the subject prevents a comprehensive examination of this field; key examples are given below.
products, the cis isomer was selected for to varying degrees.
The silicon-directed Nazarov cyclization reaction was subsequently employed in the synthesis of the natural product Silphinene, shown below. The cyclization takes place before elimination of the benzyl alcohol
moiety, so that the resulting stereochemistry
of the newly formed ring arises from approach of the silyl alkene anti to the ether.
developed a paradigm for "polarized" Nazarov cyclizations in which electron donating
and electron withdrawing groups are used to improve the overall selectivity of the reaction. Creation of an effective vinyl
nucleophile
and vinyl
electrophile
in the substrate allows catalytic activation with copper triflate and regioselective elimination. In addition, the electron withdrawing group increases the acidity of the α-proton, allowing selective formation of the trans-α-epimer via equilibration.
It is often possible to achieve catalytic activation using a donating or withdrawing group alone, although the efficiency of the reaction (yield, reaction time, etc.) is typically lower.
.
In the total synthesis
of rocaglamide, epoxidation of a vinyl alkoxyallenyl stannane likewise generates a pentadienyl cation via ring opening of the resultant epoxide
.
followed by enolate tautomerization. However, these two steps can be interrupted by various nucleophile
s and electrophile
s, respectively. Oxyallyl cation trapping has been developed extensively by Fredrick G. West of the University of Alberta
and his review covers the field. The oxyallyl cation can be trapped with heteroatom
and carbon nucleophile
s and can also undergo cationic cycloaddition
s with various tethered partners. Shown below is a cascade reaction in which successive cation trapping generates a pentacyclic core in one step with complete diastereoselectivity.
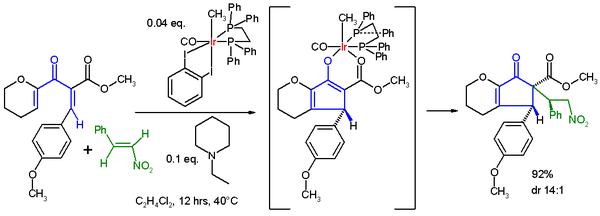
Enolate trapping with various electrophile
s is decidedly less common. In one study, the Nazarov cyclization is paired with a Michael reaction
using an iridium catalyst to initiate nucleophilic conjugate addition
of the enolate to nitrostyrene. In this tandem reaction the iridium
catalyst is required for both conversions: it acts as the Lewis acid
in the Nazarov cyclization and in the next step the nitro
group of nitrostyrene first coordinates to iridium in a ligand exchange with the carbonyl ester oxygen atom before the actual Michael addition takes place to the opposite face of the R-group.
s direct the cyclization. Almost all of the attempts are based on the idea of torquoselectivity
; selecting one direction for the vinyl groups to "rotate" in turn sets the stereochemistry as shown below.
Silicon-directed Nazarov cyclizations can exhibit induced diastereoselectivity in this way. In the example below, the silyl-group acts to direct the cyclization by preventing the distant alkene from rotating "towards" it via unfavorable steric interaction. In this way the silicon acts as a traceless auxiliary
. (The starting material is not enantiopure but the retention of enantiomeric excess
suggests that the auxiliary directs the cyclization.)
Tius's allenyl substrates can exhibit axial to tetrahedral chirality transfer if enantiopure allenes are used. The example below generates a chiral diosphenpol in 64% yield and 95% enantiomeric excess
.
Tius has additionally developed a camphor
-based auxiliary for achiral allenes that was employed in the first asymmetric synthesis of roseophilin
. The key step employs an unusual mixture of hexafluoro-2-propanol
and tetrafluoroethylene
as solvent.
The first chiral Lewis acid promoted asymmetric Nazarov cyclization was reported by Varinder Aggarwal and utilized copper (II) bisoxazoline ligand
complexes with up to 98% ee. The enantiomeric excess was unaffected by use of 50 mol% of the copper complex but the yield was significantly decreased.
can undergo a similar cationic conrotatory cyclization that is typically referred to as an iso-Nazarov cyclization reaction. Other such extensions have been given similar names, including homo
-Nazarov cyclizations and vinylogous
Nazarov cyclizations.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
used in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
for the synthesis of cyclopentenone
Cyclopentenone
Cyclopentenone is a hydrocarbon with chemical formula 56 and CAS number 930-30-3. It is structurally similar to cyclopentanone, with the additional feature of α-β unsaturation in the ring system. Cyclopentenone belongs to the cycloalkene class of compounds and is also a ketone...
s. The reaction is typically divided into classical and modern variants, depending on the reagent
Reagent
A reagent is a "substance or compound that is added to a system in order to bring about a chemical reaction, or added to see if a reaction occurs." Although the terms reactant and reagent are often used interchangeably, a reactant is less specifically a "substance that is consumed in the course of...
s and substrate
Substrate (chemistry)
In chemistry, a substrate is the chemical species being observed, which reacts with a reagent. This term is highly context-dependent. In particular, in biochemistry, an enzyme substrate is the material upon which an enzyme acts....
s employed. It was originally discovered by Ivan Nikolaevich Nazarov (1906–1957) in 1949 while studying the rearrangements of allyl vinyl ketones.

Enone
An enone is an unsaturated chemical compound or functional group consisting of a conjugated system of an alkene and a ketone. The simplest enone is methyl vinyl ketone or CH2=CHCOCH3....
using a stoichiometric Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
or protic acid promoter. The key step of the reaction mechanism involves a cationic 4π-electrocyclic ring closure
Electrocyclic reaction
In organic chemistry, an electrocyclic reaction is a type of pericyclic rearrangement reaction where the net result is one pi bond being converted into one sigma bond or vice-versa...
which forms the cyclopentenone product (See Mechanism below). As the reaction has been developed, variants involving substrates other than divinyl ketones and promoters other than Lewis acids have been subsumed under the name Nazarov cyclization provided that they follow a similar mechanistic pathway
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
.
The success of the Nazarov cyclization as a tool in organic synthesis stems from the utility and ubiquity of cyclopentenone
Cyclopentenone
Cyclopentenone is a hydrocarbon with chemical formula 56 and CAS number 930-30-3. It is structurally similar to cyclopentanone, with the additional feature of α-β unsaturation in the ring system. Cyclopentenone belongs to the cycloalkene class of compounds and is also a ketone...
s as both motifs in natural product
Natural product
A natural product is a chemical compound or substance produced by a living organism - found in nature that usually has a pharmacological or biological activity for use in pharmaceutical drug discovery and drug design...
s (including jasmone
Jasmone
Jasmone is a natural organic compound extracted from the volatile portion of the oil from jasmine flowers. It is a colorless to pale yellow liquid that has the odor of jasmine. Jasmone can exist in two isomeric forms with differing geometry around the pentenyl double bond, cis-jasmone and...
, the aflatoxin
Aflatoxin
Aflatoxins are naturally occurring mycotoxins that are produced by many species of Aspergillus, a fungus, the most notable ones being Aspergillus flavus and Aspergillus parasiticus. Aflatoxins are toxic and among the most carcinogenic substances known...
s, and a subclass of prostaglandin
Prostaglandin
A prostaglandin is any member of a group of lipid compounds that are derived enzymatically from fatty acids and have important functions in the animal body. Every prostaglandin contains 20 carbon atoms, including a 5-carbon ring....
s) and as useful synthetic intermediates for total synthesis
Total synthesis
In organic chemistry, a total synthesis is, in principle, the complete chemical synthesis of complex organic molecules from simpler pieces, usually without the aid of biological processes. In practice, these simpler pieces are commercially available in bulk and semi-bulk quantities, and are often...
. The reaction has been used in several total syntheses and several reviews have been published.
Mechanism
The mechanism of the classical Nazarov cyclization reaction was first demonstrated experimentally by Shoppe to be an intramolecularIntramolecular
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.- Examples :...
electrocyclization and is outlined below. Activation of the ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
by the acid catalyst generates a pentadienyl cation which undergoes a thermally allowed 4π conrotatory electrocyclization as dictated by the Woodward-Hoffman rules. This generates an oxyallyl cation which undergoes an elimination reaction
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
to lose a β-hydrogen. Subsequent tautomerization of the enolate produces the cyclopentenone product.

Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
groups in an appropriate orientation. The propensity of the system to enter this conformation dramatically influences reaction rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
, with α-substituted substrates having an increased population of the requisite conformer due to allylic strain
Allylic strain
thumb | 250 px | right | Allylic strain in an olefin.Allylic strain in organic chemistry is a type of strain energy resulting from the interaction between a substituent on one end of an olefin with an allylic substituent on the other end...
. Coordination of an electron donating α-substituent by the catalyst can likewise increase the reaction rate by enforcing this conformation.
Similarly, β-substitution directed inward restricts the s-trans conformation so severely that E-Z
E-Z notation
E-Z notation, or the E-Z convention, is the IUPAC preferred method of describing the stereochemistry of double bonds in organic chemistry...
isomerization has been shown to occur in advance of cyclization on a wide range of substrates, yielding the trans cyclopentenone regardless of initial configuration. In this way, the Nazarov cyclization is a rare example of a stereoselective pericyclic reaction
Pericyclic reaction
In organic chemistry, a pericyclic reaction is a type of organic reaction wherein the transition state of the molecule has a cyclic geometry, and the reaction progresses in a concerted fashion. Pericyclic reactions are usually rearrangement reactions...
, whereas most electrocyclizations are stereospecific. The example below uses triethylsilyl hydride
Reductions with hydrosilanes
Reductions with hydrosilanes are chemical reactions that involve the combination of an organosilane with an organic substrate containing unsaturated or electron-withdrawing functionality...
to trap the oxyallyl cation so that no elimination occurs. (See Interrupted cyclizations below)
Along this same vein, allenyl vinyl ketones of the type studied extensively by Marcus Tius of the University of Hawaii
University of Hawaii
The University of Hawaii System, formally the University of Hawaii and popularly known as UH, is a public, co-educational college and university system that confers associate, bachelor, master, and doctoral degrees through three university campuses, seven community college campuses, an employment...
show dramatic rate acceleration due to the removal of β-hydrogens, obviating a large amount of steric strain in the s-cis conformer.
Classical Nazarov cyclizations
Though cyclizations following the general template above had been observed prior to Nazarov's involvement, it was his study of the rearrangements of allyl vinyl ketoneKetone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s that marked the first major examination of this process. Nazarov correctly reasoned that the allylic olefin isomerized in situ to form a divinyl ketone before ring closure to the cyclopentenone product. The reaction shown below involves an alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
oxymercuration reaction
Oxymercuration reaction
The oxymercuration reaction is an electrophilic addition organic reaction that transforms an alkene into a neutral alcohol. In oxymercuration, the alkene reacts with mercuric acetate in aqueous solution to yield the addition of an acetoxymercuri group and a hydroxy group across the double bond...
to generate the requisite ketone.
Research involving the reaction was relatively quiet in subsequent years, until in the mid-1980s when several syntheses employing the Nazarov cyclization were published. Shown below are key steps in the syntheses of Trichodiene and Nor-Sterepolide, the latter of which is thought to proceed via an unusual alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
-allene
Allene
An allene is a compound in which one carbon atom has double bonds with each of its two adjacent carbon centres. Allenes are classified as polyenes with cumulated dienes. The parent compound of allene is propadiene. Compounds with an allene-type structure but with more than three carbon atoms are...
isomerization that generates the divinyl ketone.
Shortcomings
The classical version of the Nazarov cyclization suffers from several drawbacks which modern variants attempt to circumvent. The first two are not evident from the mechanism alone, but are indicative of the barriers to cyclization; the last three stem from selectivity issues relating to elimination and protonation of the intermediate.- Strong Lewis or protic acids are typically required for the reaction (e.g. TiCl4Titanium tetrachlorideTitanium tetrachloride is the inorganic compound with the formula TiCl4. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. TiCl4 is an unusual example of a metal halide that is highly volatile...
, BF3, MeSO3HMethanesulfonic acidMethanesulfonic acid is a colorless liquid with the chemical formula CH3SO3H. It is the simplest of the alkylsulfonic acids. Salts and esters of methanesulfonic acid are known as mesylates. Methanesulfonic acid is used as an acid catalyst in organic reactions because it is non-volatile, strong acid...
). These promoters are not compatible with sensitive functional groups, limiting the substrate scope. - Despite the mechanistic possibility for catalysisCatalysisCatalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
, multiple equivalentEquivalent (chemistry)The equivalent , sometimes termed the molar equivalent, is a unit of amount of substance used in chemistry and the biological sciences.The equivalent is formally defined as the amount of a substance which will either:...
s of the promoter are often required in order to effect the reaction. This limits the atom economyAtom economyAtom economy describes the conversion efficiency of a chemical process in terms of all atoms involved . In an ideal chemical process, the amount of starting materials or reactants equals the amount of all products generated and no atom is wasted...
of the reaction. - The elimination step is not regioselective; if multiple β-hydrogens are available for elimination, various products are often observed as mixtures. This is highly undesirable from an efficiency standpoint as arduous separationSeparation processIn chemistry and chemical engineering, a separation process, or simply a separation, is any mass transfer process used to convert a mixture of substances into two or more distinct product mixtures, at least one of which is enriched in one or more of the mixture's constituents. In some cases, a...
is typically required. - Elimination destroys a potential stereocenterStereocenterA stereocenter or stereogenic center is an atom, bearing groups such that an interchanging of any two groups leads to a stereoisomer.A chirality center is a stereocenter consisting of an atom holding a set of ligands in a spatial arrangement which is not superposable on its mirror image...
, decreasing the potential usefulness of the reaction. - Protonation of the enolate is sometimes not stereoselective, meaning that products can be formed as mixtures of epimerEpimerIn chemistry, epimers are diastereomers that differ in configuration of only one stereogenic center. Diastereomers are a class of stereoisomers that are non-superposable, non-mirror images of one another....
s.
Modern variants
The shortcomings noted above limit the usefulness of the Nazarov cyclization reaction in its canonical form. However, modifications to the reaction focused on remedying its issues continue to be an active area of academic research. In particular, the research has focused on a few key areas: rendering the reaction catalytic in the promoter, effecting the reaction with more mild promoters to improve functional groupFunctional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
tolerance, directing the regioselectivity
Regioselectivity
In chemistry, regioselectivity is the preference of one direction of chemical bond making or breaking over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base will abstract from an organic molecule, or where...
of the elimination step, and improving the overall stereoselectivity
Stereoselectivity
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during the non-stereospecific creation of a new stereocenter or during the non-stereospecific transformation of a pre-existing one...
. These have been successful to varying degrees.
Additionally, modifications focused on altering the progress of the reaction, either by generating the pentadienyl cation in an unorthodox fashion or by having the oxyallyl cation "intercepted" in various ways. Furthermore, enantioselective variants of various kinds have been developed. The sheer volume of literature on the subject prevents a comprehensive examination of this field; key examples are given below.
Silicon-directed cyclization
The earliest efforts to improve the selectivity of the Nazarov cyclization took advantage of the β-silicon effect in order to direct the regioselectivity of the elimination step. This chemistry was developed most extensively by Professor Scott Denmark of the University of Illinois, Urbana-Champaign in the mid-1980s and utilizes stoichiometric amounts of iron trichloride to promote the reaction. With bicyclicBicyclic molecule
A bicyclic molecule is a molecule that features two fused rings. Bicyclic molecules occur widely in organic and inorganic compounds.Fusion of the rings can occur in three ways:...
products, the cis isomer was selected for to varying degrees.
The silicon-directed Nazarov cyclization reaction was subsequently employed in the synthesis of the natural product Silphinene, shown below. The cyclization takes place before elimination of the benzyl alcohol
Benzyl alcohol
Benzyl alcohol is an organic compound with the formula C6H5CH2OH. The benzyl group is often abbreviated "Bn", thus benzyl alcohol is denoted as BnOH. Benzyl alcohol is a colorless liquid with a mild pleasant aromatic odor. It is a useful solvent due to its polarity, low toxicity, and low vapor...
moiety, so that the resulting stereochemistry
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
of the newly formed ring arises from approach of the silyl alkene anti to the ether.
Polarization
Drawing on the substituent effects compiled over various trials of the reaction, Professor Alison Frontier of the University of RochesterUniversity of Rochester
The University of Rochester is a private, nonsectarian, research university in Rochester, New York, United States. The university grants undergraduate and graduate degrees, including doctoral and professional degrees. The university has six schools and various interdisciplinary programs.The...
developed a paradigm for "polarized" Nazarov cyclizations in which electron donating
Polar effect
The Polar effect or electronic effect in chemistry is the effect exerted by a substituent on modifying electrostatic forces operating on a nearby reaction center...
and electron withdrawing groups are used to improve the overall selectivity of the reaction. Creation of an effective vinyl
Vinyl
A vinyl compound is any organic compound that contains a vinyl group ,which are derivatives of ethene, CH2=CH2, with one hydrogen atom replaced with some other group...
nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
and vinyl
Vinyl
A vinyl compound is any organic compound that contains a vinyl group ,which are derivatives of ethene, CH2=CH2, with one hydrogen atom replaced with some other group...
electrophile
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
in the substrate allows catalytic activation with copper triflate and regioselective elimination. In addition, the electron withdrawing group increases the acidity of the α-proton, allowing selective formation of the trans-α-epimer via equilibration.
It is often possible to achieve catalytic activation using a donating or withdrawing group alone, although the efficiency of the reaction (yield, reaction time, etc.) is typically lower.
Alternative cation generation
By extension, any pentadienyl cation regardless of its origin is capable of undergoing a Nazarov cyclization. There have been a large number of examples published where the requisite cation is arrived at by a variety of rearrangements. One such example involves the silver catalyzed cationic ring opening of allylic dichloro cylopropanes. The silver salt facilitates loss of chloride via precipitation of insoluble silver chlorideSilver chloride
Silver chloride is a chemical compound with the chemical formula AgCl. This white crystalline solid is well known for its low solubility in water . Upon illumination or heating, silver chloride converts to silver , which is signalled by greyish or purplish coloration to some samples...
.
In the total synthesis
Total synthesis
In organic chemistry, a total synthesis is, in principle, the complete chemical synthesis of complex organic molecules from simpler pieces, usually without the aid of biological processes. In practice, these simpler pieces are commercially available in bulk and semi-bulk quantities, and are often...
of rocaglamide, epoxidation of a vinyl alkoxyallenyl stannane likewise generates a pentadienyl cation via ring opening of the resultant epoxide
Epoxide
An epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in...
.
Interrupted cyclization
Once the cyclization has occurred, an oxyallyl cation is formed. As discussed extensively above, the typical course for this intermediate is eliminationElimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
followed by enolate tautomerization. However, these two steps can be interrupted by various nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
s and electrophile
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
s, respectively. Oxyallyl cation trapping has been developed extensively by Fredrick G. West of the University of Alberta
University of Alberta
The University of Alberta is a public research university located in Edmonton, Alberta, Canada. Founded in 1908 by Alexander Cameron Rutherford, the first premier of Alberta and Henry Marshall Tory, its first president, it is widely recognized as one of the best universities in Canada...
and his review covers the field. The oxyallyl cation can be trapped with heteroatom
Heteroatom
In organic chemistry, a heteroatom is any atom that is not carbon or hydrogen. Usually, the term is used to indicate that non-carbon atoms have replaced carbon in the backbone of the molecular structure...
and carbon nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
s and can also undergo cationic cycloaddition
Cycloaddition
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
s with various tethered partners. Shown below is a cascade reaction in which successive cation trapping generates a pentacyclic core in one step with complete diastereoselectivity.
Enolate trapping with various electrophile
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
s is decidedly less common. In one study, the Nazarov cyclization is paired with a Michael reaction
Michael reaction
The Michael reaction or Michael addition is the nucleophilic addition of a carbanion or another nucleophile to an alpha, beta unsaturated carbonyl compound. It belongs to the larger class of conjugate additions. This is one of the most useful methods for the mild formation of C-C bonds...
using an iridium catalyst to initiate nucleophilic conjugate addition
Nucleophilic conjugate addition
Nucleophilic conjugate addition is a type of organic reaction. Ordinary nucleophilic additions or 1,2-nucleophilic additions deal mostly with additions to carbonyl compounds. Simple alkene compounds do not show 1,2 reactivity due to lack of polarity, unless the alkene is activated with special...
of the enolate to nitrostyrene. In this tandem reaction the iridium
Iridium
Iridium is the chemical element with atomic number 77, and is represented by the symbol Ir. A very hard, brittle, silvery-white transition metal of the platinum family, iridium is the second-densest element and is the most corrosion-resistant metal, even at temperatures as high as 2000 °C...
catalyst is required for both conversions: it acts as the Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
in the Nazarov cyclization and in the next step the nitro
Nitro compound
Nitro compounds are organic compounds that contain one or more nitro functional groups . They are often highly explosive, especially when the compound contains more than one nitro group and is impure. The nitro group is one of the most common explosophores used globally...
group of nitrostyrene first coordinates to iridium in a ligand exchange with the carbonyl ester oxygen atom before the actual Michael addition takes place to the opposite face of the R-group.

Enantioselective variants
The development of an enantioselective Nazarov cyclization is a desirable addition to the repertoire of Nazarov cyclization reactions. To that end, several variations have been developed utilizing chiral auxiliaries and chiral catalysts. Diastereoselective cyclizations are also known, in which extant stereocenterStereocenter
A stereocenter or stereogenic center is an atom, bearing groups such that an interchanging of any two groups leads to a stereoisomer.A chirality center is a stereocenter consisting of an atom holding a set of ligands in a spatial arrangement which is not superposable on its mirror image...
s direct the cyclization. Almost all of the attempts are based on the idea of torquoselectivity
Torquoselectivity
Torquoselectivity is a special kind of stereoselectivity observed in electrocyclic reactions in organic chemistry, defined as "the preference for inward or outward rotation of substituents in conrotatory...
; selecting one direction for the vinyl groups to "rotate" in turn sets the stereochemistry as shown below.
Silicon-directed Nazarov cyclizations can exhibit induced diastereoselectivity in this way. In the example below, the silyl-group acts to direct the cyclization by preventing the distant alkene from rotating "towards" it via unfavorable steric interaction. In this way the silicon acts as a traceless auxiliary
Chiral auxiliary
A chiral auxiliary is a chemical compound or unit that is temporarily incorporated into an organic synthesis so that it can be carried out asymmetrically with the selective formation of one of two enantiomers...
. (The starting material is not enantiopure but the retention of enantiomeric excess
Enantiomeric excess
The enantiomeric excess of a substance is a measure of how pure it is. In this case, the impurity is the undesired enantiomer .-Definition:...
suggests that the auxiliary directs the cyclization.)
Tius's allenyl substrates can exhibit axial to tetrahedral chirality transfer if enantiopure allenes are used. The example below generates a chiral diosphenpol in 64% yield and 95% enantiomeric excess
Enantiomeric excess
The enantiomeric excess of a substance is a measure of how pure it is. In this case, the impurity is the undesired enantiomer .-Definition:...
.
Tius has additionally developed a camphor
Camphor
Camphor is a waxy, white or transparent solid with a strong, aromatic odor. It is a terpenoid with the chemical formula C10H16O. It is found in wood of the camphor laurel , a large evergreen tree found in Asia and also of Dryobalanops aromatica, a giant of the Bornean forests...
-based auxiliary for achiral allenes that was employed in the first asymmetric synthesis of roseophilin
Roseophilin
Roseophilin is an antibiotic isolated from Streptomyces griscovirides shown to have antitumor activity. The chemical structure can be considered in terms of two components, a macrotricyclic segment and a heterocyclic side-chain...
. The key step employs an unusual mixture of hexafluoro-2-propanol
Hexafluoro-2-propanol
Hexafluoroisopropanol, commonly abbreviated HFIP, is the organic compound with the formula 2CHOH. This fluorinated alcohol finds use as solvent and synthetic intermediate. It appears as a colorless, volatile liquid that is characterized by a strong, pungent odor...
and tetrafluoroethylene
Tetrafluoroethylene
Tetrafluoroethylene is a chemical compound with the formula C2F4. It is the simplest alkene fluorocarbon. This gaseous species is used primarily in the industrial preparation of polymers.-Properties:...
as solvent.
The first chiral Lewis acid promoted asymmetric Nazarov cyclization was reported by Varinder Aggarwal and utilized copper (II) bisoxazoline ligand
Bisoxazoline ligand
In chemistry, bisoxazoline ligands are chiral ligands based on a bis oxazoline skeleton and used in combination with a metal compound in asymmetric synthesis as a chiral catalyst . Three frequently encountered such ligands are PyBOX, tBuBOX and PhBOX...
complexes with up to 98% ee. The enantiomeric excess was unaffected by use of 50 mol% of the copper complex but the yield was significantly decreased.
Related Reactions
Extensions of the Nazarov cyclization are generally also subsumed under the same name. For example, an α-β, γ-δ unsaturated ketoneKetone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
can undergo a similar cationic conrotatory cyclization that is typically referred to as an iso-Nazarov cyclization reaction. Other such extensions have been given similar names, including homo
Homologous series
In chemistry, a homologous series is a series of compounds with a similar general formula, possessing similar chemical properties due to the presence of the same functional group, and showing a gradation in physical properties as a result of increase in molecular size and mass...
-Nazarov cyclizations and vinylogous
Vinylogous
Vinylogous is an adjective used to apply the concept of vinylogy taught in intermediate undergraduate through graduate/research organic chemistry. Vinylogy has been defined as the transmission of electronic effects through a conjugated organic bonding system...
Nazarov cyclizations.
Retro-Nazarov reaction
Because they overstabilize the pentadienyl cation, β-electron donating substiuents often severely impede Nazarov cyclization. Building from this, several electrocyclic ring openings of β-alkoxy cyclopentanes have been reported. These are typically referred to as retro-Nazarov cyclization reactions.Imino-Nazarov reaction
Nitrogen analogues of the Nazarov cyclization reaction (known as imino-Nazarov cyclization reactions) have few instances; there is one example of a generalized imino-Nazarov cyclization reported (shown below), and several iso-imino-Nazarov reactions in the literature. Even these tend to suffer from poor stereoselectivity, poor yields, or narrow scope. The difficulty stems from the relative over-stabilization of the pentadienyl cation by electron donation, impeding cyclization.See also
- Pauson-Khand reaction
- Electrocyclization
- CyclopentenoneCyclopentenoneCyclopentenone is a hydrocarbon with chemical formula 56 and CAS number 930-30-3. It is structurally similar to cyclopentanone, with the additional feature of α-β unsaturation in the ring system. Cyclopentenone belongs to the cycloalkene class of compounds and is also a ketone...
- Merrilactone AMerrilactone AMerrilactone A is one of the four sesquiterpenes that were newly discovered from the fruit of Illicium merrillianum in 2000. Members of the genus Illicium include Chinese star anise, widely used as a spice for flavouring food and beverages, and also poisonous plants such as Japanese star anise...

