
Vinylogous
Encyclopedia
Vinylogous is an adjective used to apply the concept of vinylogy taught in intermediate undergraduate through graduate/research organic chemistry
. Vinylogy has been defined as the transmission of electronic effect
s through a conjugated organic bonding system. The concept was introduced in 1926 by Ludwig Claisen to explained the acidic properties of formylacetone and related ketoaldehydes.
To be specific, the concept describes structures and reactivity that involve an atom (or group of atoms) attached via a carbon-carbon double bond (>C=C<, a "vinyl
" moiety) to a further electropositive (electron deficient) atom/group (e.g., an electron-withdrawing group, EWG, such as a carbonyl
). Vinylogous reactivity is the modified behavior observed of the atom/group that is attached via the double bond, which is therefore in conjugation with the electropositive center (EWG) — where the attached group's reactivity is observed to be analogous to reactions with the EWG itself. For instance, an hydroxyl
(-OH) group attached directly to a carbonyl is by definition a carboxylic acid
that can ionize by loss of the proton (H+) from the hydroxyl group with an equilibrium constant termed the acid's pKa
, a numerical value that reflects the acid's strength. Likewise, when a hydroxyl
group is "indirectly" attached to a carbonyl via an intervening vinyl (>C=C<) moiety, it is termed a vinylogous carboxylic acid
, and it also ionizes by loss of a proton, and with an equilibrium constant very similar to that of the analogous parent carboxylic acid (see image).
Vinylogous reactions are believed to occur when orbitals of the double bonds of the vinyl group and of the EWG group (the π orbitals) are aligned and so can overlap and mix (i.e., are conjugated). This enables the EWG to receive electron density (experience a higher probability of electron localization) through participation of the conjugated system. As noted, this reactivity is seen in vinylogous carboxylic acids, which have the >C=C(OH)- (enol
) adjacent and in conjugation with the carbonyl group (akin to the keto-enol tautomerization observed in acetylacetone
). Vinylogous reactions also include conjugate additions, where an additional electron-rich moiety (nucleophile
) reacts at the terminous of the vinyl group, as well as the vinylogous variation of the aldol reaction
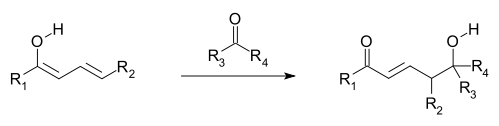
, where an additional electron-deficient moiety (electrophile
) is attacked by the nucleophile provided by a vinylogous enolate (see first and following image). The vinylogous enolate attacks with the terminal carbon of its double-bond system (the γ-carbon/position) rather than the carbon immediately adjacent to the carbonyl (the α-carbon/position, as in a simple enolate). Allylic nucleophiles often react by vinylogous nucleophilic addition instead of direct addition; these are termed allylic rearrangement
s.
As noted, behaviors in organic reactivity that are consistent with the vinylogy concept are typically explained by delocalization
of the electrons of the conjugated system
, where the π electrons of the double bond are shared with the π or lone pair
electrons in the EWG. A further acid-base example: Ascorbic acid
(vitamin C) behaves as a vinylogous carboxylic acid with involvement of its carbonyl double bond, a double bond within its ring, and the lone pair on the hydroxyl group acting as the conjugated system
. The hydroxyl proton at the terminus of the vinyl group in ascorbic acid is unusually acidic compared to a typical alcohol
-type hydroxyl group because two major resonance structures
can stabilize the negative charge on the resulting anion (conjugate base) of ascorbic acid (center and right structures in last image), analogous to the two resonance structures that stabilize the negative charge on the anion that results from removal of a proton from a simple carboxylic acid (cf. first image).
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
. Vinylogy has been defined as the transmission of electronic effect
Electronic effect
An electronic effect influences the structure, reactivity, or properties of molecule but is neither a traditional bond nor a steric effect. In organic chemistry, the term stereoelectronic effect is also used to emphasize the relation between the electronic structure and the geometry of a...
s through a conjugated organic bonding system. The concept was introduced in 1926 by Ludwig Claisen to explained the acidic properties of formylacetone and related ketoaldehydes.
To be specific, the concept describes structures and reactivity that involve an atom (or group of atoms) attached via a carbon-carbon double bond (>C=C<, a "vinyl
Vinyl
A vinyl compound is any organic compound that contains a vinyl group ,which are derivatives of ethene, CH2=CH2, with one hydrogen atom replaced with some other group...
" moiety) to a further electropositive (electron deficient) atom/group (e.g., an electron-withdrawing group, EWG, such as a carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
). Vinylogous reactivity is the modified behavior observed of the atom/group that is attached via the double bond, which is therefore in conjugation with the electropositive center (EWG) — where the attached group's reactivity is observed to be analogous to reactions with the EWG itself. For instance, an hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
(-OH) group attached directly to a carbonyl is by definition a carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
that can ionize by loss of the proton (H+) from the hydroxyl group with an equilibrium constant termed the acid's pKa
PKA
PKA, pKa, or other similar variations may stand for:* pKa, the symbol for the acid dissociation constant at logarithmic scale* Protein kinase A, a class of cAMP-dependent enzymes* Pi Kappa Alpha, the North-American social fraternity...
, a numerical value that reflects the acid's strength. Likewise, when a hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
group is "indirectly" attached to a carbonyl via an intervening vinyl (>C=C<) moiety, it is termed a vinylogous carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
, and it also ionizes by loss of a proton, and with an equilibrium constant very similar to that of the analogous parent carboxylic acid (see image).
Vinylogous reactions are believed to occur when orbitals of the double bonds of the vinyl group and of the EWG group (the π orbitals) are aligned and so can overlap and mix (i.e., are conjugated). This enables the EWG to receive electron density (experience a higher probability of electron localization) through participation of the conjugated system. As noted, this reactivity is seen in vinylogous carboxylic acids, which have the >C=C(OH)- (enol
Enol
Enols are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated anions of enols are called enolates...
) adjacent and in conjugation with the carbonyl group (akin to the keto-enol tautomerization observed in acetylacetone
Acetylacetone
Acetylacetone is an organic compound that famously exists in two tautomeric forms that rapidly interconvert. The less stable tautomer is a diketone formally named pentane-2,4-dione. The more common tautomer is the enol form. The pair of tautomers rapidly interconvert and are treated as a single...
). Vinylogous reactions also include conjugate additions, where an additional electron-rich moiety (nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
) reacts at the terminous of the vinyl group, as well as the vinylogous variation of the aldol reaction
Aldol reaction
The aldol reaction is a powerful means of forming carbon–carbon bonds in organic chemistry.Discovered independently by Charles-Adolphe Wurtz and Alexander Porfyrevich Borodin in 1872, the reaction combines two carbonyl compounds to form a new β-hydroxy carbonyl compound...
, where an additional electron-deficient moiety (electrophile
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
) is attacked by the nucleophile provided by a vinylogous enolate (see first and following image). The vinylogous enolate attacks with the terminal carbon of its double-bond system (the γ-carbon/position) rather than the carbon immediately adjacent to the carbonyl (the α-carbon/position, as in a simple enolate). Allylic nucleophiles often react by vinylogous nucleophilic addition instead of direct addition; these are termed allylic rearrangement
Allylic rearrangement
An allylic rearrangement or allylic shift is an organic reaction in which the double bond in an allyl chemical compound shifts to the next carbon atom. It is encountered in nucleophilic substitution....
s.

As noted, behaviors in organic reactivity that are consistent with the vinylogy concept are typically explained by delocalization
Delocalized electron
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or one covalent bond....
of the electrons of the conjugated system
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
, where the π electrons of the double bond are shared with the π or lone pair
Lone pair
In chemistry, a lone pair is a valence electron pair without bonding or sharing with other atoms. They are found in the outermost electron shell of an atom, so lone pairs are a subset of a molecule's valence electrons...
electrons in the EWG. A further acid-base example: Ascorbic acid
Ascorbic acid
Ascorbic acid is a naturally occurring organic compound with antioxidant properties. It is a white solid, but impure samples can appear yellowish. It dissolves well in water to give mildly acidic solutions. Ascorbic acid is one form of vitamin C. The name is derived from a- and scorbutus , the...
(vitamin C) behaves as a vinylogous carboxylic acid with involvement of its carbonyl double bond, a double bond within its ring, and the lone pair on the hydroxyl group acting as the conjugated system
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
. The hydroxyl proton at the terminus of the vinyl group in ascorbic acid is unusually acidic compared to a typical alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
-type hydroxyl group because two major resonance structures
Resonance (chemistry)
In chemistry, resonance or mesomerism is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis formula...
can stabilize the negative charge on the resulting anion (conjugate base) of ascorbic acid (center and right structures in last image), analogous to the two resonance structures that stabilize the negative charge on the anion that results from removal of a proton from a simple carboxylic acid (cf. first image).

