
Flammability limit
Encyclopedia
Flammability limits, also called flammable limits, give the proportion of combustible
gas
es in a mixture, between which limits this mixture is flammable
. Gas mixtures consisting of combustible, oxidizing
, and inert
gases are only flammable under certain conditions. The lower flammable limit
(LFL) describes the leanest mixture that still sustains a flame, i.e. the mixture with the smallest fraction of combustible gas, while the upper flammable limit (UFL) gives the richest flammable mixture.
There is a quantitative difference between flammability limits and explosive limits. In an explosive mixture the fuel oxidizer mixture is closer to stoichiometric proportion. This difference has no practical application in safety engineering
as the flammable vapor cloud is turbulent and the exact mixture of fuel and oxidizer varies greatly. Therefore, many references use the term flammability limit(LFL, UFL) and explosive limit (LEL, UEL) interchangeably.
Attaining the perfect combustible or explosive mixture between a fuel and air is important in internal combustion engine
s, for example in gasoline
or diesel
engines.
A deflagration
is a propagation of a combustion zone at a velocity less than the speed of sound in the unreacted medium. A detonation
is a propagation of a combustion zone at a velocity greater than the speed of sound in the unreacted medium. An explosion
is the bursting or rupture of an enclosure or container due to the development of internal pressure from a deflagration or detonation as defined in NFPA
69.
Methane gas has a LEL of 4.4% (at 138 degrees C) by volume, meaning 4.4% of the total volume of the air consists of methane. At 20 degrees C the LEL is 5.1 % by volume. If the atmosphere has less than 5.1% methane, an explosion cannot occur even if a source of ignition is present. When methane concentration reaches 5.1% an explosion can occur if there is an ignition source. LEL concentrations vary greatly between combustible gases.
Percentage reading on combustible air monitors should not be confused with the LEL concentrations. Explosimeter
s designed and calibrated to a specific gas may show the relative concentration of the atmosphere to the LEL - the LEL being 100%. A 5% displayed LEL reading for methane, for example, would be equivalent to 5.1% multiplied by 5%, or approximately 0.25% methane by volume at 20 degrees C. Control of the explosion hazard is usually achieved by sufficient natural or mechanical ventilation, to limit the concentration of flammable gases or vapors to a maximum level of 25% of their Lower Explosive or Flammable Limit.
's mixing rule for combustible volume fractions xi:
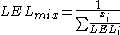
and similar for UEL.
Temperature
, pressure
, and the concentration of the oxidizer also influences flammability limits. Higher temperature results in lower LFL and higher UFL, while greater pressure increases both values. The effect of pressure is very small at pressures below 10 millibar
and difficult to predict, since it has only been studied in internal combustion engines with a turbocharger
.
Oxygen enriched atmospheres lower the LFL and increase the UFL. An atmosphere devoid of an oxidizer is neither flammable or explosive regardless of the fuel gas concentration. Increasing the fraction of inert gases in an air mixture raises the LFL and decreases the UFL.
. Methods used to control the concentration of a potentially explosive gas or vapor include use of sweep gas, an unreactive gas such as nitrogen
or argon
to dilute the explosive gas before coming in contact with air. Use of scrubbers or adsorption
resins to remove explosive gases before release are also common. Gases can also be maintained safely at concentrations above the UEL, although a breach in the storage container can lead to explosive conditions or intense fire
s.
Explosion limits also depend on the particle size of the dust involved, and are not intrinsic properties of the material. In addition, a concentration above the LEL can be created suddenly from settled dust accumulations, so management by routine monitoring, as is done with gases and vapours, is of no value. The preferred method of managing combustible dust is by preventing accumulations of settled dust through process enclosure, ventilation, and surface cleaning. However, lower explosion limits may be relevant to plant design.
Fuel
Fuel is any material that stores energy that can later be extracted to perform mechanical work in a controlled manner. Most fuels used by humans undergo combustion, a redox reaction in which a combustible substance releases energy after it ignites and reacts with the oxygen in the air...
gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
es in a mixture, between which limits this mixture is flammable
Flammability
Flammability is defined as how easily something will burn or ignite, causing fire or combustion. The degree of difficulty required to cause the combustion of a substance is quantified through fire testing. Internationally, a variety of test protocols exist to quantify flammability...
. Gas mixtures consisting of combustible, oxidizing
Oxidizing agent
An oxidizing agent can be defined as a substance that removes electrons from another reactant in a redox chemical reaction...
, and inert
Inert
-Chemistry:In chemistry, the term inert is used to describe a substance that is not chemically reactive.The noble gases were previously known as inert gases because of their perceived lack of participation in any chemical reactions...
gases are only flammable under certain conditions. The lower flammable limit
Lower flammable limit
Lower flammability limit , usually expressed in volume per cent, is the lower end of the concentration range of a flammable solvent at a given temperature and pressure for which air/vapor mixtures can ignite. The flammability range is delineated by the upper and lower flammability limit. Outside...
(LFL) describes the leanest mixture that still sustains a flame, i.e. the mixture with the smallest fraction of combustible gas, while the upper flammable limit (UFL) gives the richest flammable mixture.
There is a quantitative difference between flammability limits and explosive limits. In an explosive mixture the fuel oxidizer mixture is closer to stoichiometric proportion. This difference has no practical application in safety engineering
Safety engineering
Safety engineering is an applied science strongly related to systems engineering / industrial engineering and the subset System Safety Engineering...
as the flammable vapor cloud is turbulent and the exact mixture of fuel and oxidizer varies greatly. Therefore, many references use the term flammability limit(LFL, UFL) and explosive limit (LEL, UEL) interchangeably.
Attaining the perfect combustible or explosive mixture between a fuel and air is important in internal combustion engine
Internal combustion engine
The internal combustion engine is an engine in which the combustion of a fuel occurs with an oxidizer in a combustion chamber. In an internal combustion engine, the expansion of the high-temperature and high -pressure gases produced by combustion apply direct force to some component of the engine...
s, for example in gasoline
Gasoline
Gasoline , or petrol , is a toxic, translucent, petroleum-derived liquid that is primarily used as a fuel in internal combustion engines. It consists mostly of organic compounds obtained by the fractional distillation of petroleum, enhanced with a variety of additives. Some gasolines also contain...
or diesel
Diesel engine
A diesel engine is an internal combustion engine that uses the heat of compression to initiate ignition to burn the fuel, which is injected into the combustion chamber...
engines.
A deflagration
Deflagration
Deflagration is a term describing subsonic combustion that usually propagates through thermal conductivity; hot burning material heats the next layer of cold material and ignites it. Most "fire" found in daily life, from flames to explosions, is deflagration...
is a propagation of a combustion zone at a velocity less than the speed of sound in the unreacted medium. A detonation
Detonation
Detonation involves a supersonic exothermic front accelerating through a medium that eventually drives a shock front propagating directly in front of it. Detonations are observed in both conventional solid and liquid explosives, as well as in reactive gases...
is a propagation of a combustion zone at a velocity greater than the speed of sound in the unreacted medium. An explosion
Explosion
An explosion is a rapid increase in volume and release of energy in an extreme manner, usually with the generation of high temperatures and the release of gases. An explosion creates a shock wave. If the shock wave is a supersonic detonation, then the source of the blast is called a "high explosive"...
is the bursting or rupture of an enclosure or container due to the development of internal pressure from a deflagration or detonation as defined in NFPA
National Fire Protection Association
The National Fire Protection Association is a United States trade association that creates and maintains private, copywrited, standards and codes for usage and adoption by local governments...
69.
Lower Explosive Limit
Lower Explosive Limit (LEL): The lowest concentration (percentage) of a gas or a vapor in air capable of producing a flash of fire in presence of an ignition source (arc, flame, heat). At a concentration in air below the LEL there is not enough fuel to continue an explosion. Concentrations lower than the LEL are "too lean" to explode but may still deflagrate.Methane gas has a LEL of 4.4% (at 138 degrees C) by volume, meaning 4.4% of the total volume of the air consists of methane. At 20 degrees C the LEL is 5.1 % by volume. If the atmosphere has less than 5.1% methane, an explosion cannot occur even if a source of ignition is present. When methane concentration reaches 5.1% an explosion can occur if there is an ignition source. LEL concentrations vary greatly between combustible gases.
Percentage reading on combustible air monitors should not be confused with the LEL concentrations. Explosimeter
Explosimeter
An explosimeter is a device which is used to measure the amount of combustible gases present in a sample. When a percentage of the lower explosive limit of an atmosphere is exceeded, an alarm signal on the instrument is activated...
s designed and calibrated to a specific gas may show the relative concentration of the atmosphere to the LEL - the LEL being 100%. A 5% displayed LEL reading for methane, for example, would be equivalent to 5.1% multiplied by 5%, or approximately 0.25% methane by volume at 20 degrees C. Control of the explosion hazard is usually achieved by sufficient natural or mechanical ventilation, to limit the concentration of flammable gases or vapors to a maximum level of 25% of their Lower Explosive or Flammable Limit.
Upper Explosive Limit
Upper Explosive Limit (UEL): Highest concentration (percentage) of a gas or a vapor in air capable of producing a flash of fire in presence of an ignition source (arc, flame, heat). Concentration higher than UFL or UEL are "too rich" to burn.Influence of temperature, pressure and composition
Flammability limits of mixtures of several combustible gases can be calculated using Le ChatelierHenri Louis Le Chatelier
Henri Louis Le Châtelier was an influential French chemist of the late 19th and early 20th centuries. He is most famous for devising Le Châtelier's principle, used by chemists to predict the effect a changing condition has on a system in chemical equilibrium...
's mixing rule for combustible volume fractions xi:
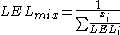
and similar for UEL.
Temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
, pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
, and the concentration of the oxidizer also influences flammability limits. Higher temperature results in lower LFL and higher UFL, while greater pressure increases both values. The effect of pressure is very small at pressures below 10 millibar
Bar (unit)
The bar is a unit of pressure equal to 100 kilopascals, and roughly equal to the atmospheric pressure on Earth at sea level. Other units derived from the bar are the megabar , kilobar , decibar , centibar , and millibar...
and difficult to predict, since it has only been studied in internal combustion engines with a turbocharger
Turbocharger
A turbocharger, or turbo , from the Greek "τύρβη" is a centrifugal compressor powered by a turbine that is driven by an engine's exhaust gases. Its benefit lies with the compressor increasing the mass of air entering the engine , thereby resulting in greater performance...
.
Oxygen enriched atmospheres lower the LFL and increase the UFL. An atmosphere devoid of an oxidizer is neither flammable or explosive regardless of the fuel gas concentration. Increasing the fraction of inert gases in an air mixture raises the LFL and decreases the UFL.
Controlling explosive atmospheres
Controlling gas and vapor concentrations outside the explosive limits is a major consideration in occupational safety and healthOccupational safety and health
Occupational safety and health is a cross-disciplinary area concerned with protecting the safety, health and welfare of people engaged in work or employment. The goal of all occupational safety and health programs is to foster a safe work environment...
. Methods used to control the concentration of a potentially explosive gas or vapor include use of sweep gas, an unreactive gas such as nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
or argon
Argon
Argon is a chemical element represented by the symbol Ar. Argon has atomic number 18 and is the third element in group 18 of the periodic table . Argon is the third most common gas in the Earth's atmosphere, at 0.93%, making it more common than carbon dioxide...
to dilute the explosive gas before coming in contact with air. Use of scrubbers or adsorption
Adsorption
Adsorption is the adhesion of atoms, ions, biomolecules or molecules of gas, liquid, or dissolved solids to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. It differs from absorption, in which a fluid permeates or is dissolved by a liquid or solid...
resins to remove explosive gases before release are also common. Gases can also be maintained safely at concentrations above the UEL, although a breach in the storage container can lead to explosive conditions or intense fire
Fire
Fire is the rapid oxidation of a material in the chemical process of combustion, releasing heat, light, and various reaction products. Slower oxidative processes like rusting or digestion are not included by this definition....
s.
Dusts
Dusts also have upper and lower explosion limits, though the upper limits are hard to measure and of little practical importance. Lower explosive limits for many organic materials are in the range of 10–50 g/m³, which is much higher than the limits set for health reasons, as is the case for the LEL of many gases and vapours. Dust clouds of this concentration are hard to see through for more than a short distance, and normally only exist inside process equipment.Explosion limits also depend on the particle size of the dust involved, and are not intrinsic properties of the material. In addition, a concentration above the LEL can be created suddenly from settled dust accumulations, so management by routine monitoring, as is done with gases and vapours, is of no value. The preferred method of managing combustible dust is by preventing accumulations of settled dust through process enclosure, ventilation, and surface cleaning. However, lower explosion limits may be relevant to plant design.
Examples
The flammable/explosive limits of some gases and vapors are given below. Concentrations are given in percent by volume of air.- Class IA liquids (Flash Point less than 73°F (22.8°C); Boiling PointBoiling pointThe boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid....
less than 100°F (37.8°C) are NFPA 704NFPA 704NFPA 704 is a standard maintained by the U.S.-based National Fire Protection Association. It defines the colloquial "fire diamond" used by emergency personnel to quickly and easily identify the risks posed by nearby hazardous materials...
Flammability Rating 4 - Classes IB (Flash Point less than 73°F (22.8°C); Boiling Point equal to or greater than 100°F (37.8°C)) and IC liquids (Flash Point equal to or greater than 73°F (22.8°C), but less than 100°F (37.8°C)) are NFPA 704 Flammability Rating 3
- Classes II (Flash Point equal to or greater than 100°F (37.8°C), but less than 140°F) and IIIA liquids (Flash Point equal to or greater than 140°F (60°C), but less than 200°F (93.3°C)) are NFPA 704 Flammability Rating 2
- Class IIIB liquids (Flash Point equal to or greater than 200°F (93.3°C) are NFPA 704 Flammability Rating 1
| Substance | LFL/LEL in % by volume of air |
UFL/UEL in % by volume of air |
NFPA NFPA NFPA may refer to:* National Fire Protection Association** NFPA 704, National Fire Protection Association Fire Diamond* National Food Processors Association* National Fluid Power Association* Non-Fossil Purchasing Agency... Class |
Flash point Flash point The flash point of a volatile material is the lowest temperature at which it can vaporize to form an ignitable mixture in air. Measuring a flash point requires an ignition source... |
Minimum Ignition Energy Minimum Ignition Energy Minimum ignition energy is the minimum amount of energy required to ignite a combustible vapor, gas or dust cloud, for example due to an electrostatic discharge. MIE is measured in joules .... in mJ expressed as percent by volume in air (Note, for many chemicals it takes the least amount of ignition energy midpoint between the LEL and UEL.) |
Autoignition Temperature |
|---|---|---|---|---|---|---|
| Acetaldehyde Acetaldehyde Acetaldehyde is an organic chemical compound with the formula CH3CHO or MeCHO. It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale industrially. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants as part... |
4.0 | 57.0 | IA | -39°C | 0.37 | 175°C |
| Acetic acid Acetic acid Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell... (glacial) |
4 | 19.9 | II | 39°C to 43°C | 463°C | |
| Acetic anhydride Acetic anhydride Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula 2O. Commonly abbreviated Ac2O, it is the simplest isolatable acid anhydride and is a widely used reagent in organic synthesis... |
II | 54°C | ||||
| Acetone Acetone Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory... |
2.6 - 3 | 12.8 - 13 | IB | -17°C | 1.15 @ 4.5% | 465°C, 485°C |
| Acetonitrile Acetonitrile Acetonitrile is the chemical compound with formula . This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture... |
IB | 2°C | 524°C | |||
| Acetyl chloride Acetyl chloride Acetyl chloride, CH3COCl, also known as ethanoyl chloride or acyl chloride, is an acid chloride derived from acetic acid. It belongs to the class of organic compounds called acyl halides. It is a colorless liquid. Acetyl chloride does not exist in nature, because contact with water would hydrolyze... |
7.3 | 19 | IB | 5°C | 390°C | |
| Acetylene Acetylene Acetylene is the chemical compound with the formula C2H2. It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in pure form and thus is usually handled as a solution.As an alkyne, acetylene is unsaturated because... |
2.5 | 82 | IA | -18°C | 0.017 @ 8.5% (in pure oxygen 0.0002 @ 40%) | 305°C |
| Acrolein Acrolein Acrolein is the simplest unsaturated aldehyde. It is produced widely but is most often immediately reacted with other products due to its instability and toxicity... |
2.8 | 31 | IB | -26°C | 0.13 | |
| Acrylonitrile Acrylonitrile Acrylonitrile is the chemical compound with the formula C3H3N. This pungent-smelling colorless liquid often appears yellow due to impurities. It is an important monomer for the manufacture of useful plastics. In terms of its molecular structure, it consists of a vinyl group linked to a nitrile... |
3.0 | 17.0 | IB | 0°C | 0.16 @ 9.0% | |
| Allyl chloride Allyl chloride Allyl chloride is the organic compound with the formula CH2=CHCH2Cl. This colorless liquid is insoluble in water but soluble in common organic solvents. It is mainly converted to epichlorohydrin, used in the production of plastics. It is a chlorinated derivative of propylene.-Production:Allyl... |
2.9 | 11.1 | IB | -32 °C | 0.77 | |
| Ammonia Ammonia Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or... |
15 | 28 | IIIB | 11°C | 680 | 651°C |
| Arsine Arsine Arsine is the chemical compound with the formula AsH3. This flammable, pyrophoric, and highly toxic gas is one of the simplest compounds of arsenic... |
4.5 - 5.1 | 78 | IA | Flammable gas | ||
| Benzene Benzene Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6.... |
1.2 | 7.8 | IB | -11°C | 0.2 @ 4.7% | 560°C |
| 1,3-Butadiene 1,3-Butadiene 1,3-Butadiene is a simple conjugated diene with the formula C4H6. It is an important industrial chemical used as a monomer in the production of synthetic rubber. When the word butadiene is used, most of the time it refers to 1,3-butadiene.... |
2.0 | 12 | IA | -85°C | 0.13 @ 5.2% | |
| Butane Butane Butane is a gas with the formula C4H10 that is an alkane with four carbon atoms. The term may refer to any of two structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, butane refers only to the unbranched n-butane isomer; the other one being called "methylpropane" or... , n-Butane |
1.6 | 8.4 | IA | -60°C | 0.25 @ 4.7% | 420 - 500°C |
| n-Butyl acetate Butyl acetate n-Butyl acetate, also known as butyl ethanoate, is an organic compound commonly used as a solvent in the production of lacquers and other products. It is also used as a synthetic fruit flavoring in foods such as candy, ice cream, cheeses, and baked goods. Butyl acetate is found in many types of... , Butyl acetate |
1 - 1.7 | 8 - 15 | IB | 24°C | 370°C | |
| Butyl alcohol, Butanol | 1 | 11 | IC | 29°C | ||
| n-Butanol N-Butanol n-Butanol or n-butyl alcohol or normal butanol is a primary alcohol with a 4-carbon structure and the molecular formula C4H9OH. Its isomers include isobutanol, 2-butanol, and tert-butanol... |
1.4 | 11.2 | IC | 35°C | 340°C | |
| n-Butyl chloride, 1-chlorobutane | 1.8 | 10.1 | IB | -6°C | 1.24 | |
| n-Butyl mercaptan | 1.4 | 10.2 | IB | 2°C | 225°C | |
| Butyl methyl ketone, 2-Hexanone | 1 | 8 | IC | 25°C | 423°C | |
| Butylene Butylene In chemistry, butylene may be an alternate name for the hydrocarbon butene, . It is also a divalent functional group with formula 2• that can be seen as the result of removing two hydrogen atoms from a butane molecule, leaving two free bonds.... , 1-Butylene, 1-Butene |
1.98 | 9.65 | IA | -80°C | ||
| Carbon disulfide Carbon disulfide Carbon disulfide is a colorless volatile liquid with the formula CS2. The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non-polar solvent... |
1.0 | 50.0 | IB | -30°C | 0.009 @ 7.8% | 90°C |
| Carbon Monoxide Carbon monoxide Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal... |
12 | 75 | IA | -191°C Flammable gas | 609°C | |
| Chlorine monoxide Chlorine monoxide Chlorine monoxide is a chemical radical with the formula ClO. It plays an important role in the process of ozone depletion. In the stratosphere, chlorine atoms react with ozone molecules to form chlorine monoxide and oxygen.... |
IA | Flammable gas | ||||
| 1-Chloro-1,1-difluoroethane 1-Chloro-1,1-difluoroethane 1-Chloro-1,1-difluoroethane, also known by trade names including Freon 142b is a haloalkane with the chemical formula CH3CClF2. It is primarily used as arefrigerant.... |
6.2 | 17.9 | IA | -65°C Flammable Gas | ||
| Cyanogen Cyanogen Cyanogen is the chemical compound with the formula 2. It is a colorless, toxic gas with a pungent odor.The molecule is a pseudohalogen. Cyanogen molecules consist of two CN groups — analogous to diatomic halogen molecules, such as Cl2, but far less oxidizing... |
6.0 - 6.6 | 32 - 42.6 | IA | Flammable gas | ||
| Cyclobutane Cyclobutane Cyclobutane is an organic compound with the formula 4. Cyclobutane is a colourless gas and commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes... |
1.8 | 11.1 | IA | -63.9°C | 426.7°C | |
| Cyclohexane Cyclohexane Cyclohexane is a cycloalkane with the molecular formula C6H12. Cyclohexane is used as a nonpolar solvent for the chemical industry, and also as a raw material for the industrial production of adipic acid and caprolactam, both of which being intermediates used in the production of nylon... |
1.3 | 7.8 - 8 | IB | -18°C - -20°C | 0.22 @ 3.8% | 245°C |
| Cyclohexanol Cyclohexanol Cyclohexanol is the organic compound with the formula 5CHOH. The molecule is related to cyclohexane ring by replacement of one hydrogen atom by a hydroxyl group. This compound exists as a deliquescent colorless solid, which, when very pure, melts near room temperature... |
1 | 9 | IIIA | 68°C | 300°C | |
| Cyclohexanone Cyclohexanone Cyclohexanone is the organic compound with the formula 5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oil has an odor reminiscent of peardrop sweets as well as acetone. Over time, samples assume a yellow color due to oxidation... |
1 - 1.1 | 9 - 9.4 | II | 43.9 - 44°C | 420°C | |
| Cyclopentadiene Cyclopentadiene Cyclopentadiene is an organic compound with the formula C5H6. This colorless liquid has a strong and unpleasant odor. At room temperature, this cyclic diene dimerizes over the course of hours to give dicyclopentadiene via a Diels–Alder reaction... |
IB | 0°C | 0.67 | 640°C | ||
| Cyclopentane Cyclopentane Cyclopentane is a highly flammable alicyclic hydrocarbon with chemical formula 510 and CAS number 287-92-3, consisting of a ring of five carbon atoms each bonded with two hydrogen atoms above and below the plane. It occurs as a colorless liquid with a petrol-like odor. Its melting point is −94 °C... |
1.5 - 2 | 9.4 | IB | - 37 to -38.9°C | 0.54 | 361°C |
| Cyclopropane Cyclopropane Cyclopropane is a cycloalkane molecule with the molecular formula C3H6, consisting of three carbon atoms linked to each other to form a ring, with each carbon atom bearing two hydrogen atoms... |
2.4 | 10.4 | IA | -94.4°C | 0.17 @ 6.3% | 498°C |
| Decane Decane Decane is an alkane hydrocarbon with the chemical formula CH38CH3.75 structural isomers of decane exist, all of which are flammable liquids. Decane is one of the components of gasoline . Like other alkanes, it is nonpolar and therefore will not dissolve in polar liquids such as water... |
0.8 | 5.4 | II | 46.1°C | 210°C | |
| Diborane Diborane Diborane is the chemical compound consisting of boron and hydrogen with the formula B2H6. It is a colorless gas at room temperature with a repulsively sweet odor. Diborane mixes well with air, easily forming explosive mixtures. Diborane will ignite spontaneously in moist air at room temperature... |
0.8 | 88 | IA | -90°C Flammable gas | 38°C | |
| o-Dichlorobenzene, 1,2-Dichlorobenzene | 2 | 9 | IIIA | 65°C | 648°C | |
| 1,1-Dichloroethane 1,1-Dichloroethane 1,1-Dichloroethane is a chlorinated hydrocarbon. It is a colorless oily liquid with a chloroform-like odor. It is not easily soluble in water, but miscible with most organic solvents.... |
6 | 11 | IB | 14°C | ||
| 1,2-Dichloroethane 1,2-Dichloroethane The chemical compound 1,2-dichloroethane, commonly known by its old name of ethylene dichloride , is a chlorinated hydrocarbon, mainly used to produce vinyl chloride monomer , the major precursor for PVC production. It is a colourless liquid with a chloroform-like odour... |
6 | 16 | IB | 13°C | 413°C | |
| 1,1-Dichloroethene 1,1-Dichloroethene 1,1-Dichloroethene, commonly called 1,1-dichloroethylene or 1,1-DCE, is an organochloride with the molecular formula C2H2Cl2. It is a colorless liquid with a sharp odor. Like most chlorocarbons, it is poorly soluble in water, but soluble in organic solvents... |
6.5 | 15.5 | IA | -10°C Flammable gas | ||
| Dichlorofluoromethane Dichlorofluoromethane Dichlorofluoromethane or Freon 21 or R 21 is a halomethane or hydrochlorofluorocarbon. It is a colorless and odorless gas.Its critical point is at 178.5 °C and 517 MPa... |
54.7 | Non flammable, -36.1°C | 552°C | |||
| Dichloromethane Dichloromethane Dichloromethane is an organic compound with the formula CH2Cl2. This colorless, volatile liquid with a moderately sweet aroma is widely used as a solvent. Although it is not miscible with water, it is miscible with many organic solvents... , Methylene chloride |
16 | 66 | Non flammable | |||
| Dichlorosilane Dichlorosilane Dichlorosilane , or DCS as it is commonly known, is usually mixed with ammonia in LPCVD chambers to grow silicon nitride in semiconductor processing.A higher concentration of DCS:NH3 Dichlorosilane (H2SiCl2), or DCS as it is commonly known, is usually mixed with ammonia (NH3) in LPCVD chambers to... |
4 - 4.7 | 96 | IA | -28 °C | 0.015 | |
| Diesel fuel | 0.6 | 7.5 | IIIA | >62°C (143°F) | 210°C | |
| Diethanolamine | 2 | 13 | IB | 169°C | ||
| Diethylamine Diethylamine Diethylamine is a secondary amine with the molecular structure CH3CH2NHCH2CH3. It is a flammable, strongly alkaline liquid. It is miscible with water and ethanol. It is a colorless liquid which often appears brown due to impurities... |
1.8 | 10.1 | IB | -23°C to -26°C | 312°C | |
| Diethyl disulfide | 1.2 | II | 38.9°C | |||
| Diethyl ether Diethyl ether Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor... |
1.9 - 2 | 36 - 48 | IA | -45°C | 0.19 @ 5.1% | 160 - 170°C |
| Diethyl sulfide Diethyl sulfide Diethyl sulfide is a clear flammable chemical compound with a pungent garlic-like odor. It has the chemical formula , though it can also be written or to make its chemical structure more clear... |
IB | -10°C | ||||
| 1,1-Difluoroethane | 3.7 | 18 | IA | -81.1°C | ||
| 1,1-Difluoroethylene | 5.5 | 21.3 | -126.1°C | |||
| Diisobutyl ketone | 1 | 6 | 49°C | |||
| Diisopropyl ether Diisopropyl ether Diisopropyl ether is secondary ether that is used as a solvent. It is a colorless liquid that is slightly soluble in water, but miscible with most organic solvents... |
1 | 21 | IB | -28°C | ||
| Dimethylamine Dimethylamine Dimethylamine is an organic compound with the formula 2NH. This secondary amine is a colorless, flammable liquified gas with an ammonia-like odor. Dimethylamine is generally encountered as a solution in water at concentrations up to around 40%... |
2.8 | 14.4 | IA | Flammable gas | ||
| 1,1-Dimethylhydrazine | IB | |||||
| Dimethyl sulfide Dimethyl sulfide Dimethyl sulfide or methylthiomethane is an organosulfur compound with the formula 2S. Dimethyl sulfide is a water-insoluble flammable liquid that boils at and has a characteristic disagreeable odor. It is a component of the smell produced from cooking of certain vegetables, notably maize,... |
IA | -49°C | ||||
| Dimethyl sulfoxide Dimethyl sulfoxide Dimethyl sulfoxide is an organosulfur compound with the formula 2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water... |
2.6 - 3 | 42 | IIIB | 88 - 95°C | 215°C | |
| 1,4-Dioxane 1,4-Dioxane 1,4-Dioxane, often called dioxane because the other isomers of dioxane are rare, is a heterocyclic organic compound. It is a colorless liquid with a faint sweet odor similar to that of diethyl ether. It is classified as an ether. This colorless liquid is mainly used as a stabilizer for the solvent... |
2 | 22 | IB | 12°C | ||
| Epichlorohydrin Epichlorohydrin Epichlorohydrin is an organochlorine compound and an epoxide. This is a colorless liquid with a pungent, garlic-like odor, moderately soluble in water, but miscible with most polar organic solvents. Epichlorohydrin is a highly reactive compound and is used in the production of glycerol, plastics,... |
4 | 21 | 31°C | |||
| Ethane Ethane Ethane is a chemical compound with chemical formula C2H6. It is the only two-carbon alkane that is an aliphatic hydrocarbon. At standard temperature and pressure, ethane is a colorless, odorless gas.... |
3 | 12 - 12.4 | IA | Flammable gas -135 °C | 515°C | |
| Ethanol Ethanol Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a... , Ethyl Alcohol |
3 - 3.3 | 19 | IB | 12.8°C (55°F) | 365°C | |
| 2-Ethoxyethanol 2-Ethoxyethanol 2-Ethoxyethanol, also known by the trademark Cellosolve or ethyl cellosolve, is a solvent used widely in commercial and industrial applications... |
3 | 18 | 43°C | |||
| 2-Ethoxyethyl acetate | 2 | 8 | 56°C | |||
| Ethyl acetate Ethyl acetate Ethyl acetate is the organic compound with the formula CH3COOCH2CH3. This colorless liquid has a characteristic sweet smell and is used in glues, nail polish removers, and cigarettes... |
2 | 12 | IA | -4°C | 460°C | |
| Ethylamine Ethylamine Ethylamine is an organic compound with the formula CH3CH2NH2. This colourless gas has a strong ammonia-like odor. It is miscible with virtually all solvents and is considered to be a weak base, as is typical for amines. Ethylamine is widely used in chemical industry and organic... |
3.5 | 14 | IA | -17 °C | ||
| Ethylbenzene Ethylbenzene Ethylbenzene is an organic compound with the formula C6H5CH2CH3. This aromatic hydrocarbon is important in the petrochemical industry as an intermediate in the production of styrene, which in turn is used for making polystyrene, a common plastic material.... |
1.0 | 7.1 | 15-20 °C | |||
| Ethylene Ethylene Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone... |
2.7 | 36 | IA | 0.07 | 490°C | |
| Ethylene glycol Ethylene glycol Ethylene glycol is an organic compound widely used as an automotive antifreeze and a precursor to polymers. In its pure form, it is an odorless, colorless, syrupy, sweet-tasting liquid... |
3 | 22 | 111°C | |||
| Ethylene oxide Ethylene oxide Ethylene oxide, also called oxirane, is the organic compound with the formula . It is a cyclic ether. This means that it is composed of two alkyl groups attached to an oxygen atom in a cyclic shape . This colorless flammable gas with a faintly sweet odor is the simplest epoxide, a three-membered... |
3 | 100 | IA | −20 °C | ||
| Ethyl Chloride | 3.8 | 15.4 | IA | −50°C | ||
| Ethyl Mercaptan | IA | |||||
| Fuel oil No.1 | 0.7 | 5 | ||||
| Furan Furan Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen. The class of compounds containing such rings are also referred to as furans.... |
2 | 14 | IA | -36°C | ||
| Gasoline Gasoline Gasoline , or petrol , is a toxic, translucent, petroleum-derived liquid that is primarily used as a fuel in internal combustion engines. It consists mostly of organic compounds obtained by the fractional distillation of petroleum, enhanced with a variety of additives. Some gasolines also contain... (100 Octane Octane Octane is a hydrocarbon and an alkane with the chemical formula C8H18, and the condensed structural formula CH36CH3. Octane has many structural isomers that differ by the amount and location of branching in the carbon chain... ) |
1.4 | 7.6 | IB | < −40°C (−40°F) | 246 - 280°C | |
| Glycerol Glycerol Glycerol is a simple polyol compound. It is a colorless, odorless, viscous liquid that is widely used in pharmaceutical formulations. Glycerol has three hydroxyl groups that are responsible for its solubility in water and its hygroscopic nature. The glycerol backbone is central to all lipids... |
3 | 19 | 199°C | |||
| Heptane Heptane n-Heptane is the straight-chain alkane with the chemical formula H3C5CH3 or C7H16. When used as a test fuel component in anti-knock test engines, a 100% heptane fuel is the zero point of the octane rating scale... , n-Heptane |
1.05 | 6.7 | -4°C | 0.24 @ 3.4% | 204 - 215°C | |
| Hexane Hexane Hexane is a hydrocarbon with the chemical formula C6H14; that is, an alkane with six carbon atoms.The term may refer to any of four other structural isomers with that formula, or to a mixture of them. In the IUPAC nomenclature, however, hexane is the unbranched isomer ; the other four structures... , n-Hexane |
1.1 | 7.5 | -22°C | 0.24 @ 3.8% | 225°C, 233°C | |
| Hydrogen Hydrogen Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly... , dihydrogen, molecular H with two protons together |
4/17 | 75/56 | IA | Flammable gas | 0.016 @ 28% (in pure oxygen 0.0012) | 500 - 571°C |
| Hydrogen sulfide Hydrogen sulfide Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million... |
4.3 | 46 | IA | Flammable gas | 0.068 | |
| Isobutane Isobutane Isobutane, also known as methylpropane, is an isomer of butane. It is the simplest alkane with a tertiary carbon. Concerns with depletion of the ozone layer by freon gases have led to increased use of isobutane as a gas for refrigeration systems, especially in domestic refrigerators and freezers,... |
1.8 | 9.6 | IA | Flammable gas | 462°C | |
| Isobutyl alcohol | 2 | 11 | 28°C | |||
| Isophorone Isophorone Isophorone is an α,β-Unsaturated cyclic ketone, a colorless to yellowish liquid with characteristic smell, that is used as a solvent and as an intermediate in organic synthesis... |
1 | 4 | 84°C | |||
| Isopropyl alcohol Isopropyl alcohol Isopropyl alcohol is a common name for a chemical compound with the molecular formula C3H8O. It is a colorless, flammable chemical compound with a strong odor... , Isopropanol |
2 | 12 | IB | 12°C | 398 - 399°C; 425°C | |
| Isopropyl chloride Isopropyl chloride Isopropyl chloride is a colorless, flammable chemical compound . It has the chemical formula C3H7Cl and is prepared by refluxing isopropyl alcohol with concentrated hydrochloric acid and zinc chloride... |
IA | |||||
| Kerosene Kerosene Kerosene, sometimes spelled kerosine in scientific and industrial usage, also known as paraffin or paraffin oil in the United Kingdom, Hong Kong, Ireland and South Africa, is a combustible hydrocarbon liquid. The name is derived from Greek keros... Jet A-1 |
0.6 - 0.7 | 4.9 - 5 | II | >38°C (100°F) as jet fuel | 210°C | |
| Lithium Hydride Lithium hydride Lithium hydride is the inorganic compound with the formula LiH. It is a colorless solid, although commercial samples are gray. Characteristic of a salt-like, or ionic, hydride, it has a high melting point and is not soluble in any solvent with which it does not react... |
IA | |||||
| 2-Mercaptoethanol 2-Mercaptoethanol 2-Mercaptoethanol is the chemical compound with the formula HOCH2CH2SH. It is a hybrid of ethylene glycol, HOCH2CH2OH, and 1,2-ethanedithiol, HSCH2CH2SH... |
IIIA | |||||
| Methane Methane Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel... (Natural Gas) |
4.4 - 5 | 15 - 17 | IA | Flammable gas | 0.21 @ 8.5% | 580°C |
| Methyl acetate Methyl acetate Methyl acetate, also known as MeOAc, acetic acid methyl ester or methyl ethanoate, is a carboxylate ester with the formula CH3COOCH3. It is normally a flammable liquid with a characteristic, pleasant smell like certain glues or nail polish removers. Methyl acetate has characteristics very similar... |
3 | 16 | -10°C | |||
| Methyl Alcohol, Methanol | 6 - 6.7 | 36 | IB | 11°C | 385°C; 455°C | |
| Methylamine Methylamine Methylamine is the organic compound with a formula of CH3NH2. This colourless gas is a derivative of ammonia, but with one H atom replaced by a methyl group. It is the simplest primary amine. It is sold as a solution in methanol, ethanol, THF, and water, or as the anhydrous gas in pressurized... |
IA | 8°C | ||||
| Methyl Chloride | 10.7 | 17.4 | IA | -46 °C | ||
| Methyl ether | IA | −41 °C | ||||
| Methyl ethyl ether | IA | |||||
| Methyl ethyl ketone | 1.8 | 10 | IB | -6°C | 505 - 515°C | |
| Methyl formate Methyl formate Methyl formate, also called methyl methanoate, is the methyl ester of formic acid. The simplest example of an ester, it is a clear liquid with an ethereal odor, high vapor pressure, and low surface tension.-Production:... |
IA | |||||
| Methyl mercaptan | 3.9 | 21.8 | IA | -53°C | ||
| Mineral spirits | 0.7 | 6.5 | 38-43°C | 258°C | ||
| Morpholine Morpholine Morpholine is an organic chemical compound having the chemical formula O2NH. This heterocycle, pictured at right, features both amine and ether functional groups. Because of the amine, morpholine is a base; its conjugate acid is called morpholinium... |
1.8 | 10.8 | IC | 31 - 37.7°C | 310°C | |
| Naphthalene Naphthalene Naphthalene is an organic compound with formula . It is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings... |
0.9 | 5.9 | IIIA | 79 - 87 °C | ||
| Neohexane | 1.19 | 7.58 | −29 °C | 425°C | ||
| Nickel tetracarbonyl | 2 | 34 | 4 °C | 60 °C | ||
| Nitrobenzene Nitrobenzene Nitrobenzene is an organic compound with the chemical formula C6H5NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale as a precursor to aniline. Although occasionally used as a flavoring or perfume... |
2 | 9 | IIIA | 88°C | ||
| Nitromethane Nitromethane Nitromethane is an organic compound with the chemical formula . It is the simplest organic nitro compound. It is a slightly viscous, highly polar liquid commonly used as a solvent in a variety of industrial applications such as in extractions, as a reaction medium, and as a cleaning solvent... |
7.3 | 22.2 | 35°C | 379°C | ||
| Octane Octane Octane is a hydrocarbon and an alkane with the chemical formula C8H18, and the condensed structural formula CH36CH3. Octane has many structural isomers that differ by the amount and location of branching in the carbon chain... |
1 | 7 | 13°C | |||
| iso-Octane | 0.79 | 5.94 | ||||
| Pentane Pentane Pentane is an organic compound with the formula C5H12 — that is, an alkane with five carbon atoms. The term may refer to any of three structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, pentane means exclusively the n-pentane isomer; the other two being called... |
1.5 | 7.8 | IA | -40 to -49°C | as 2-Pentane 0.18 @ 4.4% | 260°C |
| n-Pentane | 1.4 | 7.8 | IA | 0.28 @ 3.3% | ||
| iso-Pentane | 1.32 | 9.16 | IA | 420°C | ||
| Phosphine Phosphine Phosphine is the compound with the chemical formula PH3. It is a colorless, flammable, toxic gas. Pure phosphine is odourless, but technical grade samples have a highly unpleasant odor like garlic or rotting fish, due to the presence of substituted phosphine and diphosphine... |
IA | |||||
| Propane Propane Propane is a three-carbon alkane with the molecular formula , normally a gas, but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is commonly used as a fuel for engines, oxy-gas torches, barbecues, portable stoves, and residential central... |
2.1 | 9.5 - 10.1 | IA | Flammable gas | 0.25 @ 5.2% (in pure oxygen 0.0021) | 480°C |
| Propyl acetate Propyl acetate The chemical compound propyl acetate, also known as propyl ethanoate, is a common solvent. This clear, colourless liquid is known by its characteristic odour of pears. Due to this fact, it is commonly used as a flavouring additive... |
2 | 8 | 13°C | |||
| Propylene Propylene Propene, also known as propylene or methylethylene, is an unsaturated organic compound having the chemical formula C3H6. It has one double bond, and is the second simplest member of the alkene class of hydrocarbons, and it is also second in natural abundance.-Properties:At room temperature and... |
2.0 | 11.1 | IA | -108°C | 0.28 | 458°C |
| Propylene Oxide Propylene oxide Propylene oxide is an organic compound with the molecular formula CH3CHCH2O. This colourless volatile liquid is produced on a large scale industrially, its major application being its use for the production of polyether polyols for use in making polyurethane plastics... |
2.3 | 36 | IA | |||
| Pyridine Pyridine Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom... |
2 | 12 | 20 | |||
| Silane Silane Silane is a toxic, extremely flammable chemical compound with chemical formula SiH4. In 1857, the German chemists and Friedrich Woehler discovered silane among the products formed by the action of hydrochloric acid on aluminum silicide, which they had previously prepared... |
1.5 | 98 | IA | <21°C | ||
| Styrene | 1.1 | 6.1 | IB | 31 - 32.2°C | 490°C | |
| Tetrafluoroethylene Tetrafluoroethylene Tetrafluoroethylene is a chemical compound with the formula C2F4. It is the simplest alkene fluorocarbon. This gaseous species is used primarily in the industrial preparation of polymers.-Properties:... |
IA | |||||
| Tetrahydrofuran Tetrahydrofuran Tetrahydrofuran is a colorless, water-miscible organic liquid with low viscosity at standard temperature and pressure. This heterocyclic compound has the chemical formula 4O. As one of the most polar ethers with a wide liquid range, it is a useful solvent. Its main use, however, is as a precursor... |
2 | 12 | IB | -14°C | 321°C | |
| Toluene Toluene Toluene, formerly known as toluol, is a clear, water-insoluble liquid with the typical smell of paint thinners. It is a mono-substituted benzene derivative, i.e., one in which a single hydrogen atom from the benzene molecule has been replaced by a univalent group, in this case CH3.It is an aromatic... |
1.2 -1.27 | 6.75 - 7.1 | IB | 4.4°C | 0.24 @ 4.1% | 480°C; 535°C |
| Triethylborane Triethylborane Triethylborane , also called triethylborine and triethylboron, is an organoborane , a near-colorless to yellowish transparent liquid with pungent ether-like odor. Its chemical formula can be written as C6H15B, or 3B, or 3B, or Et3B.Triethylborane is strongly pyrophoric, igniting spontaneously in... |
-20°C | -20°C | ||||
| Trimethylamine Trimethylamine Trimethylamine is an organic compound with the formula N3. This colorless, hygroscopic, and flammable tertiary amine has a strong "fishy" odor in low concentrations and an ammonia-like odor at higher concentrations... |
IA | Flammable gas | ||||
| Trinitrobenzene | IA | |||||
| Turpentine Turpentine Turpentine is a fluid obtained by the distillation of resin obtained from trees, mainly pine trees. It is composed of terpenes, mainly the monoterpenes alpha-pinene and beta-pinene... |
0.8 | IC | 35°C | |||
| Vegetable oil | IIIB | 327°C (620°F) | ||||
| Vinyl acetate Vinyl acetate Vinyl acetate is an organic compound with the formula CH3COOCH=CH2. A colorless liquid with a pungent odor, it is the precursor to polyvinyl acetate, an important polymer in industry.-Production:... |
2.6 | 13.4 | −8 °C | |||
| Vinyl chloride Vinyl chloride Vinyl chloride is the organochloride with the formula H2C:CHCl. It is also called vinyl chloride monomer, VCM or chloroethene. This colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride . At ambient pressure and temperature, vinyl chloride... |
3.6 | 33 | ||||
| Xylene Xylene Xylene encompasses three isomers of dimethylbenzene. The isomers are distinguished by the designations ortho- , meta- , and para- , which specify to which carbon atoms the two methyl groups are attached... s |
0.9 - 1.0 | 6.7 - 7.0 | IC | 27 - 32°C | 0.2 | |
| m-Xylene M-Xylene m-Xylene is an aromatic hydrocarbon, based on benzene with two methyl substituents.It is an isomer of o-xylene and p-xylene. The m stands for meta, meaning the two methyl substituents are at locants 1 and 3 on the aromatic ring.... |
1.1 | 7 | IC | 25°C | 525°C | |
| o-Xylene O-Xylene o-Xylene is an aromatic hydrocarbon, based on benzene with two methyl substituents bonded to adjacent carbon atoms in the aromatic ring .It is a constitutional isomer of m-xylene and p-xylene.... |
IC | 17 °C | ||||
| p-Xylene P-Xylene p-Xylene is an aromatic hydrocarbon, based on benzene with two methyl substituents. The “p” stands for para, identifying the location of the methyl groups as across from one another.... |
1.0 | 6.0 | IC | 27.2°C | 530°C |
Further reading
- David R. Lide, Editor-in-Chief; CRC Handbook of Chemistry and Physics, 72nd edition; CRC Press; Boca Raton, FloridaBoca Raton, FloridaBoca Raton is a city in Palm Beach County, Florida, USA, incorporated in May 1925. In the 2000 census, the city had a total population of 74,764; the 2006 population recorded by the U.S. Census Bureau was 86,396. However, the majority of the people under the postal address of Boca Raton, about...
; 1991; ISBN 0-8493-0565-9

