2-Mercaptoethanol
Encyclopedia
2-Mercaptoethanol is the chemical compound
with the formula
HOCH2CH2SH. It is a hybrid of ethylene glycol
, HOCH2CH2OH, and 1,2-ethanedithiol
, HSCH2CH2SH. ME or βME, as it is commonly abbreviated, is used to reduce disulfide bonds and can act as a biological antioxidant
by scavenging hydroxyl radicals (amongst others). It is widely used because the hydroxyl group confers solubility in water and lowers the volatility. Due to its diminished vapor pressure, its odour, while unpleasant, is less objectionable than related thiol
s.
on ethylene oxide
:
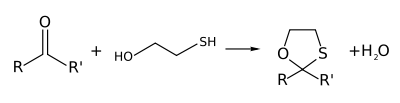
s and ketone
s to give the corresponding oxathiolanes. This makes 2-mercaptoethanol useful as a protecting group
.
By breaking the S-S bonds, both the tertiary structure
and the quaternary structure
of some proteins can be disrupted. Because of its ability to disrupt the structure of proteins, it was used in the analysis of proteins, for instance, to ensure that a protein solution contains monomeric protein molecules, instead of disulfide linked dimers or higher order oligomers. However, since 2-mercaptoethanol forms adducts with free cysteines and is somewhat more toxic, dithiothreitol
(DTT) is more generally employed especially in SDS-PAGE
. DTT is also a more powerful reducing agent with a redox potential (at pH 7) of -0.33 V, compared to -0.26 V for 2-mercaptoethanol.
2-mercaptoethanol is often used interchangeably with dithiothreitol
(DTT) or the odorless tris(2-carboxyethyl)phosphine (TCEP
) in biological applications.
2-mercaptoethanol is more stable than DTT (2-ME: t1/2>100h at pH6.5, t1/2=4h at pH8.5; DTT: t1/2=40h at pH6.5, t1/2=1.5h at pH8.5; Stevens et al., 1983), but has a higher volatility.
released during cell lysis. Numerous disulfide bonds make ribonucleases very stable enzymes, so 2-mercaptoethanol is used to reduce these disulfide bonds and irreversibly denature the proteins. This prevents them from digesting the RNA during its extraction procedure.
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
with the formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
HOCH2CH2SH. It is a hybrid of ethylene glycol
Ethylene glycol
Ethylene glycol is an organic compound widely used as an automotive antifreeze and a precursor to polymers. In its pure form, it is an odorless, colorless, syrupy, sweet-tasting liquid...
, HOCH2CH2OH, and 1,2-ethanedithiol
1,2-Ethanedithiol
1,2-Ethanedithiol is a colorless liquid with the formula C2H42. It has a very characteristic odor which is compared by many people to rotten cabbage. It is a common building block in organic synthesis and an excellent ligand for metal ions.-Preparation:...
, HSCH2CH2SH. ME or βME, as it is commonly abbreviated, is used to reduce disulfide bonds and can act as a biological antioxidant
Antioxidant
An antioxidant is a molecule capable of inhibiting the oxidation of other molecules. Oxidation is a chemical reaction that transfers electrons or hydrogen from a substance to an oxidizing agent. Oxidation reactions can produce free radicals. In turn, these radicals can start chain reactions. When...
by scavenging hydroxyl radicals (amongst others). It is widely used because the hydroxyl group confers solubility in water and lowers the volatility. Due to its diminished vapor pressure, its odour, while unpleasant, is less objectionable than related thiol
Thiol
In organic chemistry, a thiol is an organosulfur compound that contains a carbon-bonded sulfhydryl group...
s.
Preparation
2-Mercaptoethanol may be prepared by the action of hydrogen sulfideHydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
on ethylene oxide
Ethylene oxide
Ethylene oxide, also called oxirane, is the organic compound with the formula . It is a cyclic ether. This means that it is composed of two alkyl groups attached to an oxygen atom in a cyclic shape . This colorless flammable gas with a faintly sweet odor is the simplest epoxide, a three-membered...
:
Reactions
2-Mercaptoethanol reacts with aldehydeAldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s and ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s to give the corresponding oxathiolanes. This makes 2-mercaptoethanol useful as a protecting group
Protecting group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group in order to obtain chemoselectivity in a subsequent chemical reaction...
.
Reducing proteins
Some proteins can be denatured by 2-mercaptoethanol via its ability to cleave disulfide bonds:- cysCystineCystine is a dimeric amino acid formed by the oxidation of two cysteine residues that covalently link to make a disulfide bond. This organosulfur compound has the formula 2. It is a white solid, and melts at 247-249 °C...
S-Scys + 2 HOCH2CH2SH → 2 cysSHCysteineCysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
+ HOCH2CH2S-SCH2CH2OH
By breaking the S-S bonds, both the tertiary structure
Tertiary structure
In biochemistry and molecular biology, the tertiary structure of a protein or any other macromolecule is its three-dimensional structure, as defined by the atomic coordinates.-Relationship to primary structure:...
and the quaternary structure
Quaternary structure
In biochemistry, quaternary structure is the arrangement of multiple folded protein or coiling protein molecules in a multi-subunit complex.-Description and examples:...
of some proteins can be disrupted. Because of its ability to disrupt the structure of proteins, it was used in the analysis of proteins, for instance, to ensure that a protein solution contains monomeric protein molecules, instead of disulfide linked dimers or higher order oligomers. However, since 2-mercaptoethanol forms adducts with free cysteines and is somewhat more toxic, dithiothreitol
Dithiothreitol
Dithiothreitol is the common name for a small-molecule redox reagent known as Cleland's reagent. DTT's formula is C4H10O2S2 and the molecular structure of its reduced form is shown at the right; its oxidized form is a disulfide-bonded 6-membered ring . Its name derives from the four-carbon...
(DTT) is more generally employed especially in SDS-PAGE
SDS-PAGE
SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis, describes a collection of related techniques widely used in biochemistry, forensics, genetics and molecular biology to separate proteins according to their electrophoretic mobility...
. DTT is also a more powerful reducing agent with a redox potential (at pH 7) of -0.33 V, compared to -0.26 V for 2-mercaptoethanol.
2-mercaptoethanol is often used interchangeably with dithiothreitol
Dithiothreitol
Dithiothreitol is the common name for a small-molecule redox reagent known as Cleland's reagent. DTT's formula is C4H10O2S2 and the molecular structure of its reduced form is shown at the right; its oxidized form is a disulfide-bonded 6-membered ring . Its name derives from the four-carbon...
(DTT) or the odorless tris(2-carboxyethyl)phosphine (TCEP
TCEP
TCEP is a reducing agent frequently used in biochemistry and molecular biology applications. It is often prepared and used as a hydrochloride salt with a molecular weight of 286.65 gram/mol...
) in biological applications.
2-mercaptoethanol is more stable than DTT (2-ME: t1/2>100h at pH6.5, t1/2=4h at pH8.5; DTT: t1/2=40h at pH6.5, t1/2=1.5h at pH8.5; Stevens et al., 1983), but has a higher volatility.
Preventing protein oxidation
2-Mercaptoethanol and related reducing agents (e.g., DTT) are often included in enzymatic reactions to inhibit the oxidation of free sulfhydryl residues, and hence maintain protein activity.Denaturing ribonucleases
2-Mercaptoethanol is used in some RNA isolation procedures to eliminate ribonucleaseRibonuclease
Ribonuclease is a type of nuclease that catalyzes the degradation of RNA into smaller components. Ribonucleases can be divided into endoribonucleases and exoribonucleases, and comprise several sub-classes within the EC 2.7 and 3.1 classes of enzymes.-Function:All organisms studied contain...
released during cell lysis. Numerous disulfide bonds make ribonucleases very stable enzymes, so 2-mercaptoethanol is used to reduce these disulfide bonds and irreversibly denature the proteins. This prevents them from digesting the RNA during its extraction procedure.