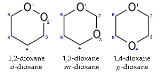
1,4-Dioxane
Encyclopedia
1,4-Dioxane, often called dioxane because the other isomer
s of dioxane are rare, is a heterocyclic organic compound
. It is a colorless liquid
with a faint sweet odor
similar to that of diethyl ether
. It is classified as an ether
. This colorless liquid is mainly used as a stabilizer for the solvent
trichloroethane
. It is an occasionally used solvent for a variety of practical applications as well as in the laboratory.
, which in turn arises from the hydrolysis of ethylene oxide
. The molecule is centrosymmetric, meaning that it adopts a chair conformation, typical of relatives of cyclohexane
. The molecule is conformationally flexible, and the boat conformation is easily adopted, as required for chelation to metal cations. In 1985, the global production capacity for dioxane was between 11,000 and 14,000 tons. In 1990, the total U.S. production volume of dioxane was between 10,500,000 and 18,300,000 pounds.
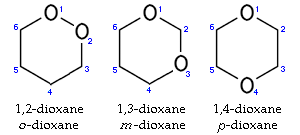
Dioxane has three isomers: in addition to 1,4-dioxane, 1,2-dioxane
and 1,3-dioxane
are also known.
for storage and transport in aluminium containers. Normally aluminium is protected by a passivating oxide layer, but when these layers are disturbed, highly reactive metallic aluminium is exposed to the chlorocarbon. This aluminium reacts with 1,1,1-trichloroethane to give aluminium trichloride, which in turn catalyses the dehydrohalogenation
of the remaining 1,1,1-trichloroethane to vinylidene chloride and hydrogen chloride
. Reflecting its properties as a ligand
, dioxane "poisons" the aluminum trichloride catalyst, by formation of an adduct
. Apart from its use as a stabilizer, dioxane is used in a variety of applications as a solvent, e.g. in inks and adhesives.
. Diethyl ether is rather insoluble in water, whereas dioxane is miscible and in fact is hygroscopic. At standard pressure, the mixture of water and dioxane in the ratio 17.9:82.1 by mass is a positive azeotrope
that boils at 87.6°C. Dioxane is a versatile polar aprotic solvent. The oxygen atom is Lewis basic, so it is able to solvate many inorganic compounds. Because of its lower toxicity, it is substituted for tetrahydrofuran
(THF) in some processes. However, it has a higher boiling point (101 °C versus 66 °C for THF), which is important when reactions are to be conducted at a higher temperature.
The oxygen centres are Lewis basic and so dioxane serves as a chelating diether ligand. It reacts with Grignard reagent
s to precipitate the magnesium dihalide. In this way, dioxane is used to drive the Schlenk equilibrium
. Dimethylmagnesium
is prepared in this manner:
It is used as an internal standard for proton NMR spectroscopy in D2O.
as a Group 2B carcinogen: possibly carcinogenic to humans because it is a known carcinogen in animals. The U.S. Environmental Protection Agency classifies dioxane as a probable human carcinogen
(having observed an increased incidence of cancer in controlled animal studies, but not in epidemiological studies of workers using the compound), and a known irritant (with a no-observed-adverse-effects level of 400 milligrams per cubic meter) at concentrations significantly higher than those found in commercial products. Under Proposition 65, dioxane is classified in the U.S. state of California to cause cancer. Dioxane is toxic to rats.
Like some other ethers, dioxane combines with atmospheric oxygen on standing to form explosive peroxide
s. Distillation
of dioxanes concentrates these peroxides increasing the danger.
process, a route to some ingredients found in cleansing and moisturizing products, dioxane can contaminate cosmetics and personal care products such as deodorants, shampoos, toothpastes and mouthwashes. The ethoxylation process makes the cleansing agents, such as sodium lauryl sulfate, less abrasive and offers enhanced foaming characteristics. 1,4-Dioxane is found in small amounts in some cosmetics, a yet unregulated substance used in cosmetics in both China and the U.S.
In 2008, testing sponsored by the U.S. Organic Consumers Association found dioxane in almost half of tested organic personal-care products. Since 1979 the U.S. Food and Drug Administration (FDA) have conducted tests on cosmetic raw materials and finished products for the levels of 1,4-dioxane. 1,4-Dioxane was present in ethoxylated raw ingredients at levels up to 1410 ppm, and at levels up to 279 ppm in off the shelf cosmetic products. Levels of 1,4-dioxane exceeding 85 ppm in children's shampoos indicate that close monitoring of raw materials and finished products is warranted. While the FDA encourages manufacturers to remove 1,4-dioxane, it is not required by federal law. A number of health authorities around the world have banned imports of products containing 1,4-dioxane.
Isomer
In chemistry, isomers are compounds with the same molecular formula but different structural formulas. Isomers do not necessarily share similar properties, unless they also have the same functional groups. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical...
s of dioxane are rare, is a heterocyclic organic compound
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
. It is a colorless liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
with a faint sweet odor
Odor
An odor or odour is caused by one or more volatilized chemical compounds, generally at a very low concentration, that humans or other animals perceive by the sense of olfaction. Odors are also commonly called scents, which can refer to both pleasant and unpleasant odors...
similar to that of diethyl ether
Diethyl ether
Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
. It is classified as an ether
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
. This colorless liquid is mainly used as a stabilizer for the solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
trichloroethane
Trichloroethane
Trichloroethane can refer to either of two isomeric chemical compounds:* 1,1,1-Trichloroethane * 1,1,2-Trichloroethane...
. It is an occasionally used solvent for a variety of practical applications as well as in the laboratory.
Synthesis and structure
Dioxane is produced by the acid-catalysed dehydration of diethylene glycolDiethylene glycol
Diethylene glycol is an organic compound with the formula 2O. It is a colorless, practically odorless, poisonous, and hygroscopic liquid with a sweetish taste. It is miscible in water, alcohol, ether, acetone, and ethylene glycol. DEG is a widely used solvent...
, which in turn arises from the hydrolysis of ethylene oxide
Ethylene oxide
Ethylene oxide, also called oxirane, is the organic compound with the formula . It is a cyclic ether. This means that it is composed of two alkyl groups attached to an oxygen atom in a cyclic shape . This colorless flammable gas with a faintly sweet odor is the simplest epoxide, a three-membered...
. The molecule is centrosymmetric, meaning that it adopts a chair conformation, typical of relatives of cyclohexane
Cyclohexane
Cyclohexane is a cycloalkane with the molecular formula C6H12. Cyclohexane is used as a nonpolar solvent for the chemical industry, and also as a raw material for the industrial production of adipic acid and caprolactam, both of which being intermediates used in the production of nylon...
. The molecule is conformationally flexible, and the boat conformation is easily adopted, as required for chelation to metal cations. In 1985, the global production capacity for dioxane was between 11,000 and 14,000 tons. In 1990, the total U.S. production volume of dioxane was between 10,500,000 and 18,300,000 pounds.
Dioxane has three isomers: in addition to 1,4-dioxane, 1,2-dioxane
1,2-Dioxane
1,2-Dioxane or o-dioxane is a chemical compound with the molecular formula C4H8O2....
and 1,3-dioxane
1,3-Dioxane
1,3-Dioxane or m-dioxane is a chemical compound with the molecular formula C4H8O2.1,3-Dioxanes and 1,3-dioxolanes are prepared from carbonyl compounds with 1,3-propanediol or 1,2-ethanediol in the presence of Brönsted or Lewis acid catalysts....
are also known.

Uses
Dioxane is primarily used as a stabilizer for 1,1,1-trichloroethane1,1,1-Trichloroethane
The organic compound 1,1,1-trichloroethane, also known as methyl chloroform, is a chloroalkane. This colourless, sweet-smelling liquid was once produced industrially in large quantities for use as a solvent...
for storage and transport in aluminium containers. Normally aluminium is protected by a passivating oxide layer, but when these layers are disturbed, highly reactive metallic aluminium is exposed to the chlorocarbon. This aluminium reacts with 1,1,1-trichloroethane to give aluminium trichloride, which in turn catalyses the dehydrohalogenation
Dehydrohalogenation
Dehydrohalogenation is an organic reaction from which an alkene is obtained from an alkyl halide . It is also called a β-Elimination reaction and is a type of elimination reaction....
of the remaining 1,1,1-trichloroethane to vinylidene chloride and hydrogen chloride
Hydrogen chloride
The compound hydrogen chloride has the formula HCl. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric humidity. Hydrogen chloride gas and hydrochloric acid are important in technology and industry...
. Reflecting its properties as a ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
, dioxane "poisons" the aluminum trichloride catalyst, by formation of an adduct
Adduct
An adduct is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all components. The resultant is considered a distinct molecular species...
. Apart from its use as a stabilizer, dioxane is used in a variety of applications as a solvent, e.g. in inks and adhesives.
Solvent properties
Dioxane is relatively nonpolar but has superior dissolving power relative to diethyl etherDiethyl ether
Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
. Diethyl ether is rather insoluble in water, whereas dioxane is miscible and in fact is hygroscopic. At standard pressure, the mixture of water and dioxane in the ratio 17.9:82.1 by mass is a positive azeotrope
Azeotrope
An azeotrope is a mixture of two or more liquids in such a ratio that its composition cannot be changed by simple distillation. This occurs because, when an azeotrope is boiled, the resulting vapor has the same ratio of constituents as the original mixture....
that boils at 87.6°C. Dioxane is a versatile polar aprotic solvent. The oxygen atom is Lewis basic, so it is able to solvate many inorganic compounds. Because of its lower toxicity, it is substituted for tetrahydrofuran
Tetrahydrofuran
Tetrahydrofuran is a colorless, water-miscible organic liquid with low viscosity at standard temperature and pressure. This heterocyclic compound has the chemical formula 4O. As one of the most polar ethers with a wide liquid range, it is a useful solvent. Its main use, however, is as a precursor...
(THF) in some processes. However, it has a higher boiling point (101 °C versus 66 °C for THF), which is important when reactions are to be conducted at a higher temperature.
The oxygen centres are Lewis basic and so dioxane serves as a chelating diether ligand. It reacts with Grignard reagent
Grignard reaction
The Grignard reaction is an organometallic chemical reaction in which alkyl- or aryl-magnesium halides add to a carbonyl group in an aldehyde or ketone. This reaction is an important tool for the formation of carbon–carbon bonds...
s to precipitate the magnesium dihalide. In this way, dioxane is used to drive the Schlenk equilibrium
Schlenk equilibrium
The Schlenk equilibrium is a chemical equilibrium named after its discoverer Wilhelm Schlenk taking place in solutions of Grignard reagents.The process described is an equilibrium between two equivalents of an alkyl or aryl magnesium halide on the left of the equation and on the right side, one...
. Dimethylmagnesium
Dimethylmagnesium
Dimethylmagnesium is an organomagnesium compound. Like other dialkylmagnesium compounds, it is prepared by adding at least one equivalent of dioxane to a solution of methylmagnesium halide:...
is prepared in this manner:
- 2 CH3MgBr + (C2H4O)2 → MgBr2(C2H4O)2 + (CH3)2Mg
It is used as an internal standard for proton NMR spectroscopy in D2O.
Safety
Dioxane is a relatively nontoxic substance with an of 5170 mg/kg. This compound is irritating to the eyes and respiratory tract. It is suspected of causing damage to the central nervous system, liver and kidneys. Accidental worker exposure to 1,4-dioxane has resulted in several deaths. Dioxane is classified by the IARCInternational Agency for Research on Cancer
The International Agency for Research on Cancer is an intergovernmental agency forming part of the World Health Organisation of the United Nations....
as a Group 2B carcinogen: possibly carcinogenic to humans because it is a known carcinogen in animals. The U.S. Environmental Protection Agency classifies dioxane as a probable human carcinogen
Carcinogen
A carcinogen is any substance, radionuclide, or radiation that is an agent directly involved in causing cancer. This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes...
(having observed an increased incidence of cancer in controlled animal studies, but not in epidemiological studies of workers using the compound), and a known irritant (with a no-observed-adverse-effects level of 400 milligrams per cubic meter) at concentrations significantly higher than those found in commercial products. Under Proposition 65, dioxane is classified in the U.S. state of California to cause cancer. Dioxane is toxic to rats.
Like some other ethers, dioxane combines with atmospheric oxygen on standing to form explosive peroxide
Peroxide
A peroxide is a compound containing an oxygen–oxygen single bond or the peroxide anion .The O−O group is called the peroxide group or peroxo group. In contrast to oxide ions, the oxygen atoms in the peroxide ion have an oxidation state of −1.The simplest stable peroxide is hydrogen peroxide...
s. Distillation
Distillation
Distillation is a method of separating mixtures based on differences in volatilities of components in a boiling liquid mixture. Distillation is a unit operation, or a physical separation process, and not a chemical reaction....
of dioxanes concentrates these peroxides increasing the danger.
Environment
Dioxane at the level of 1 μg/L has been detected in many locations in the US. In the State of New Hampshire alone in 2010 it had been found at 67 sites, ranging in concentration from 2 ppb to over 11,000 ppb. Thirty of these sites are solid waste landfills, most of which have been closed for years. It also has low toxicity to aquatic life and can be biodegraded via a number of pathways. Dioxane has affected groundwater supplies in several areas. The problems are exacerbated since dioxane is highly soluble in water, does not readily bind to soils, and readily leaches to groundwater. It is also resistant to naturally occurring biodegradation processes. Due to these properties, a dioxane plume is often much larger (and further downgradient) than the associated solvent plume.Cosmetics
As a byproduct of the ethoxylationEthoxylation
Ethoxylation is an industrial process in which ethylene oxide is added to alcohols and phenols to give surfactants. The invention of the process is attributed to Schöller and Wittwer at I.G. Farben industrie.-Production:...
process, a route to some ingredients found in cleansing and moisturizing products, dioxane can contaminate cosmetics and personal care products such as deodorants, shampoos, toothpastes and mouthwashes. The ethoxylation process makes the cleansing agents, such as sodium lauryl sulfate, less abrasive and offers enhanced foaming characteristics. 1,4-Dioxane is found in small amounts in some cosmetics, a yet unregulated substance used in cosmetics in both China and the U.S.
In 2008, testing sponsored by the U.S. Organic Consumers Association found dioxane in almost half of tested organic personal-care products. Since 1979 the U.S. Food and Drug Administration (FDA) have conducted tests on cosmetic raw materials and finished products for the levels of 1,4-dioxane. 1,4-Dioxane was present in ethoxylated raw ingredients at levels up to 1410 ppm, and at levels up to 279 ppm in off the shelf cosmetic products. Levels of 1,4-dioxane exceeding 85 ppm in children's shampoos indicate that close monitoring of raw materials and finished products is warranted. While the FDA encourages manufacturers to remove 1,4-dioxane, it is not required by federal law. A number of health authorities around the world have banned imports of products containing 1,4-dioxane.
See also
- 1,2-dioxane1,2-Dioxane1,2-Dioxane or o-dioxane is a chemical compound with the molecular formula C4H8O2....
- 1,3-dioxane1,3-Dioxane1,3-Dioxane or m-dioxane is a chemical compound with the molecular formula C4H8O2.1,3-Dioxanes and 1,3-dioxolanes are prepared from carbonyl compounds with 1,3-propanediol or 1,2-ethanediol in the presence of Brönsted or Lewis acid catalysts....
- Crown etherCrown etherCrown ethers are cyclic chemical compounds that consist of a ring containing several ether groups. The most common crown ethers are oligomers of ethylene oxide, the repeating unit being ethyleneoxy, i.e., -CH2CH2O-. Important members of this series are the tetramer , the pentamer , and the hexamer...
- Sodium laureth sulfateSodium laureth sulfateSodium laureth sulfate, or sodium lauryl ether sulfate , is a detergent and surfactant found in many personal care products . SLES is an inexpensive and very effective foaming agent. SLES, SLS and ALS are surfactants that are used in many cosmetic products for their cleansing and emulsifying...
- Oxalic anhydrideOxalic anhydrideOxalic anhydride or ethanedioic anhydride, also called oxiranedione, is a hypothetical organic compound with the formula C2O3, which can be viewed as the anhydride of oxalic acid or the two-fold ketone of ethylene oxide. It is an oxide of carbon .The simple compound apparently has yet to be...
- Dioxane tetraketoneDioxane tetraketoneDioxane tetraketone is an organic compound with the formula C4O6. It is an oxide of carbon , which can be viewed as the fourfold ketone of dioxane...

