
Isobutane
Encyclopedia
Isobutane, also known as methylpropane, is an isomer
of butane
. It is the simplest alkane
with a tertiary carbon
. Concerns with depletion of the ozone layer
by freon gases have led to increased use of isobutane as a gas for refrigeration
systems, especially in domestic refrigerator
s and freezers, and as a propellant
in aerosol spray
s. When used as a refrigerant
or a propellant, isobutane is also known as R-600a. Some portable camp stoves use a mixture of isobutane with propane
, usually 80:20. Isobutane is used as a feedstock in the petrochemical
industry, for example in the synthesis of isooctane.
Its UN number (for hazardous substances see shipping) is UN 1969.
Isobutane is the R group for the amino acid
leucine
.
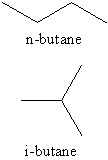 Isobutane is the trivial name
Isobutane is the trivial name
retained by the International Union of Pure and Applied Chemistry
(IUPAC) in its 1993 Recommendations for the Nomenclature of Organic Chemistry.
Since the longest continuous chain in isobutane is only three carbon atoms, the systematic name is methylpropane. The position number (2-) is unnecessary because it is the only possibility in methylpropane.
The use in refrigerators started in 1993 when Greenpeace presented the Greenfreeze project with the German company Foron.
Blends of pure, dry "isobutane" (R-600a) (commercial term used to describe isobutane mixtures) have negligible ozone depletion potential
and very low Global Warming Potential
(having a value of 3.3 times the GWP of carbon dioxide) and can serve as a functional replacement for R-12
, R-22
, R-134a
, and other chlorofluorocarbon
or hydrofluorocarbon refrigerant
s in conventional stationary refrigeration and air conditioning systems.
. Although unclear how serious this could be, at the time this report came out it was estimated 300 million refrigerators worldwide use isobutane as a refrigerant.
Although these stories are only speculation, the use of a flammable gas as a refrigerant is quite dangerous and encompasses a great deal of risk. The normal risks a CFC or other toxic refrigerant would have when it escapes, are mainly related to depletion of breathable air and frosting at the point of escape. Isobutane has an explosion risk associated also (in addition to depletion of breathable air and frosting). This explosion risk is more dangerous directly to anyone in the vicinity should it accumulate and come into contact with any ignition source. To reduce the risk, the charge of refrigerators was reduced by more than 50% against R134a. The typical charge of a R600a freezer is 45g and of a refrigerator 20g. If 45g are lost at once, 1m³ of explosive gas can be created. The risk that all gas is lost outside in a short time is very low. For interior refrigeration applications, the R600a appliances should have a hidden condenser, so that the gas can not be lost. 20g is for example approximately 3-4 times the charge of a cigarette lighter. In Europe the limit is 150g for R600a applinces due to safety.
According to an MSDS (Material Safety Data Sheet) for isobutane, this gas should not be exposed to temperatures above 52 Celsius or 151 Fahrenheit while in a closed system such as a storage tank. The range of flammability also factors into safety of this substance as a refrigerant. Flammable ranges for isobutane are between 1.8% - 8.4% and this creates a hazard when a leak forms in the refrigeration system. Many sources of ignition may be nearby so the unit could possibly explode due to the gas leak and cause major damage, not to mention injuries or death to persons nearby. This will create a problem for refrigeration technicians if a leak is reported. The leak must be checked in open air or well ventilated environments and the technician could risk injury to themselves or others. In essence, the refrigeration unit would need full replacement in most cases.
Substitution of this refrigerant for motor vehicle air conditioning systems not originally designed for R600a is widely prohibited or discouraged, on the grounds that using flammable
hydrocarbons in systems originally designed to carry non-flammable refrigerant presents a significant risk of fire or explosion.
Vendors and advocates of hydrocarbon refrigerants argue against such bans on the grounds that there have been very few such incidents relative to the number of vehicle air conditioning systems filled with hydrocarbons. One particular test was conducted by a professor at the University of New South Wales that unintentionally tested the scenario of a sudden and complete refrigerant loss into the passenger compartment followed by subsequent ignition. He and several others in the car sustained burns to their face, ears, and hands, and several observers received lacerations from the burst glass of the front passenger window.
Isomer
In chemistry, isomers are compounds with the same molecular formula but different structural formulas. Isomers do not necessarily share similar properties, unless they also have the same functional groups. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical...
of butane
Butane
Butane is a gas with the formula C4H10 that is an alkane with four carbon atoms. The term may refer to any of two structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, butane refers only to the unbranched n-butane isomer; the other one being called "methylpropane" or...
. It is the simplest alkane
Alkane
Alkanes are chemical compounds that consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds without any cycles...
with a tertiary carbon
Carbon-carbon bond
A carbon–carbon bond is a covalent bond between two carbon atoms. The most common form is the single bond: a bond composed of two electrons, one from each of the two atoms. The carbon–carbon single bond is a sigma bond and is said to be formed between one hybridized orbital from each...
. Concerns with depletion of the ozone layer
Ozone layer
The ozone layer is a layer in Earth's atmosphere which contains relatively high concentrations of ozone . This layer absorbs 97–99% of the Sun's high frequency ultraviolet light, which is potentially damaging to the life forms on Earth...
by freon gases have led to increased use of isobutane as a gas for refrigeration
Refrigeration
Refrigeration is a process in which work is done to move heat from one location to another. This work is traditionally done by mechanical work, but can also be done by magnetism, laser or other means...
systems, especially in domestic refrigerator
Refrigerator
A refrigerator is a common household appliance that consists of a thermally insulated compartment and a heat pump that transfers heat from the inside of the fridge to its external environment so that the inside of the fridge is cooled to a temperature below the ambient temperature of the room...
s and freezers, and as a propellant
Propellant
A propellant is a material that produces pressurized gas that:* can be directed through a nozzle, thereby producing thrust ;...
in aerosol spray
Aerosol spray
Aerosol spray is a type of dispensing system which creates an aerosol mist of liquid particles. This is used with a can or bottle that contains a liquid under pressure. When the container's valve is opened, the liquid is forced out of a small hole and emerges as an aerosol or mist...
s. When used as a refrigerant
Refrigerant
A refrigerant is a substance used in a heat cycle usually including, for enhanced efficiency, a reversible phase change from a liquid to a gas. Traditionally, fluorocarbons, especially chlorofluorocarbons, were used as refrigerants, but they are being phased out because of their ozone depletion...
or a propellant, isobutane is also known as R-600a. Some portable camp stoves use a mixture of isobutane with propane
Propane
Propane is a three-carbon alkane with the molecular formula , normally a gas, but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is commonly used as a fuel for engines, oxy-gas torches, barbecues, portable stoves, and residential central...
, usually 80:20. Isobutane is used as a feedstock in the petrochemical
Petrochemical
Petrochemicals are chemical products derived from petroleum. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as corn or sugar cane....
industry, for example in the synthesis of isooctane.
Its UN number (for hazardous substances see shipping) is UN 1969.
Isobutane is the R group for the amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
leucine
Leucine
Leucine is a branched-chain α-amino acid with the chemical formula HO2CCHCH2CH2. Leucine is classified as a hydrophobic amino acid due to its aliphatic isobutyl side chain. It is encoded by six codons and is a major component of the subunits in ferritin, astacin and other 'buffer' proteins...
.
Nomenclature
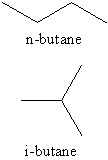
Trivial name
In chemistry, a trivial name is a common name or vernacular name; it is a non-systematic name or non-scientific name. That is, the name is not recognised according to the rules of any formal system of nomenclature...
retained by the International Union of Pure and Applied Chemistry
International Union of Pure and Applied Chemistry
The International Union of Pure and Applied Chemistry is an international federation of National Adhering Organizations that represents chemists in individual countries. It is a member of the International Council for Science . The international headquarters of IUPAC is located in Zürich,...
(IUPAC) in its 1993 Recommendations for the Nomenclature of Organic Chemistry.
Since the longest continuous chain in isobutane is only three carbon atoms, the systematic name is methylpropane. The position number (2-) is unnecessary because it is the only possibility in methylpropane.
Uses
- As a refrigerantRefrigerantA refrigerant is a substance used in a heat cycle usually including, for enhanced efficiency, a reversible phase change from a liquid to a gas. Traditionally, fluorocarbons, especially chlorofluorocarbons, were used as refrigerants, but they are being phased out because of their ozone depletion...
.
The use in refrigerators started in 1993 when Greenpeace presented the Greenfreeze project with the German company Foron.
Blends of pure, dry "isobutane" (R-600a) (commercial term used to describe isobutane mixtures) have negligible ozone depletion potential
Ozone depletion potential
The ozone depletion potential of a chemical compound is the relative amount of degradation to the ozone layer it can cause, with trichlorofluoromethane being fixed at an ODP of 1.0. Chlorodifluoromethane , for example, has an ODP of 0.055...
and very low Global Warming Potential
Global warming potential
Global-warming potential is a relative measure of how much heat a greenhouse gas traps in the atmosphere. It compares the amount of heat trapped by a certain mass of the gas in question to the amount of heat trapped by a similar mass of carbon dioxide. A GWP is calculated over a specific time...
(having a value of 3.3 times the GWP of carbon dioxide) and can serve as a functional replacement for R-12
Dichlorodifluoromethane
Dichlorodifluoromethane , is a colorless gas, and usually sold under the brand name Freon-12, is a chlorofluorocarbon halomethane , used as a refrigerant and aerosol spray propellant. Complying with the Montreal Protocol, its manufacture was banned in the United States along with many other...
, R-22
Chlorodifluoromethane
Chlorodifluoromethane or difluoromonochloromethane is a hydrochlorofluorocarbon . This colorless gas is better known as HCFC-22, or R-22. It was once commonly used as a propellant and in air conditioning applications...
, R-134a
1,1,1,2-Tetrafluoroethane
1,1,1,2-Tetrafluoroethane, R-134a, Genetron 134a, Suva 134a or HFC-134a, is a haloalkane refrigerant with thermodynamic properties similar to R-12 , but with less ozone depletion potential...
, and other chlorofluorocarbon
Chlorofluorocarbon
A chlorofluorocarbon is an organic compound that contains carbon, chlorine, and fluorine, produced as a volatile derivative of methane and ethane. A common subclass are the hydrochlorofluorocarbons , which contain hydrogen, as well. They are also commonly known by the DuPont trade name Freon...
or hydrofluorocarbon refrigerant
Refrigerant
A refrigerant is a substance used in a heat cycle usually including, for enhanced efficiency, a reversible phase change from a liquid to a gas. Traditionally, fluorocarbons, especially chlorofluorocarbons, were used as refrigerants, but they are being phased out because of their ozone depletion...
s in conventional stationary refrigeration and air conditioning systems.
- As a propellant for aerosol cans and foamFoam-Definition:A foam is a substance that is formed by trapping gas in a liquid or solid in a divided form, i.e. by forming gas regions inside liquid regions, leading to different kinds of dispersed media...
products.
Safety Concerns
Reports surfaced in late 2009 suggesting the use of isobutane as a refrigerant in domestic refrigerators was potentially dangerous. Several explosions resulting from the isobutane leaking into the refrigerator cabinet and a spark from the electrical system have been reported in the United KingdomUnited Kingdom
The United Kingdom of Great Britain and Northern IrelandIn the United Kingdom and Dependencies, other languages have been officially recognised as legitimate autochthonous languages under the European Charter for Regional or Minority Languages...
. Although unclear how serious this could be, at the time this report came out it was estimated 300 million refrigerators worldwide use isobutane as a refrigerant.
Although these stories are only speculation, the use of a flammable gas as a refrigerant is quite dangerous and encompasses a great deal of risk. The normal risks a CFC or other toxic refrigerant would have when it escapes, are mainly related to depletion of breathable air and frosting at the point of escape. Isobutane has an explosion risk associated also (in addition to depletion of breathable air and frosting). This explosion risk is more dangerous directly to anyone in the vicinity should it accumulate and come into contact with any ignition source. To reduce the risk, the charge of refrigerators was reduced by more than 50% against R134a. The typical charge of a R600a freezer is 45g and of a refrigerator 20g. If 45g are lost at once, 1m³ of explosive gas can be created. The risk that all gas is lost outside in a short time is very low. For interior refrigeration applications, the R600a appliances should have a hidden condenser, so that the gas can not be lost. 20g is for example approximately 3-4 times the charge of a cigarette lighter. In Europe the limit is 150g for R600a applinces due to safety.
According to an MSDS (Material Safety Data Sheet) for isobutane, this gas should not be exposed to temperatures above 52 Celsius or 151 Fahrenheit while in a closed system such as a storage tank. The range of flammability also factors into safety of this substance as a refrigerant. Flammable ranges for isobutane are between 1.8% - 8.4% and this creates a hazard when a leak forms in the refrigeration system. Many sources of ignition may be nearby so the unit could possibly explode due to the gas leak and cause major damage, not to mention injuries or death to persons nearby. This will create a problem for refrigeration technicians if a leak is reported. The leak must be checked in open air or well ventilated environments and the technician could risk injury to themselves or others. In essence, the refrigeration unit would need full replacement in most cases.
Substitution of this refrigerant for motor vehicle air conditioning systems not originally designed for R600a is widely prohibited or discouraged, on the grounds that using flammable
Flammability
Flammability is defined as how easily something will burn or ignite, causing fire or combustion. The degree of difficulty required to cause the combustion of a substance is quantified through fire testing. Internationally, a variety of test protocols exist to quantify flammability...
hydrocarbons in systems originally designed to carry non-flammable refrigerant presents a significant risk of fire or explosion.
Vendors and advocates of hydrocarbon refrigerants argue against such bans on the grounds that there have been very few such incidents relative to the number of vehicle air conditioning systems filled with hydrocarbons. One particular test was conducted by a professor at the University of New South Wales that unintentionally tested the scenario of a sudden and complete refrigerant loss into the passenger compartment followed by subsequent ignition. He and several others in the car sustained burns to their face, ears, and hands, and several observers received lacerations from the burst glass of the front passenger window.
External links
- International Chemical Safety Card 0901
- NIOSH Pocket Guide to Chemical Hazards
- IUPAC Nomenclature of Organic Chemistry (online version of the "Blue Book")
- Data from Air Liquide

