
Sharpless epoxidation
Encyclopedia
The Sharpless Epoxidation reaction is an enantioselective
chemical reaction
to prepare 2,3-epoxyalcohols from primary and secondary allylic alcohol
s.
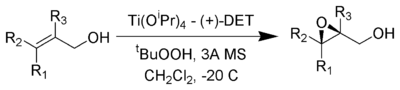 The stereochemistry of the resulting epoxide is determined by the diastereomer of the chiral tartrate diester (usually diethyl tartrate
The stereochemistry of the resulting epoxide is determined by the diastereomer of the chiral tartrate diester (usually diethyl tartrate
or diisopropyl tartrate
) employed in the reaction. The oxidizing agent is tert-butyl hydroperoxide
. Enantioselectivity is achieved by a catalyst formed from titanium tetra(isopropoxide) and diethyl tartrate. Only 5-10 mol% of the catalyst in the presence of 3Å
molecular sieve
s (3Å MS) is necessary.
The Sharpless Epoxidation's success is due to five major reasons. First, epoxides can be easily converted into diol
s, aminoalcohols or ether
s, so formation of chiral epoxides is a very important step in the synthesis of natural products. Second, the Sharpless Epoxidation reacts with many primary and secondary allylic alcohols. Third, the products of the Sharpless Epoxidation frequently have enantiomeric excess
es above 90%. Fourth, the products of the Sharpless Epoxidation are predictable using the Sharpless Epoxidation model. Finally, the reactants for the Sharpless Epoxidation are commercially available and relatively cheap.
Several reviews have been published.
K. Barry Sharpless
shared the 2001 Nobel prize in Chemistry
for his work on asymmetric oxidations.
The prize was shared with William S. Knowles and Ryōji Noyori
.
The product of allylic 1,2-diols is incorrectly predicted by this model.
of a racemic mixture of secondary 2,3-epoxyalcohols. While the yield of a kinetic resolution process cannot be higher than 50%, the enantiomeric excess
approaches 100% in some reactions.
To demonstrate the synthetic utility of the Sharpless Epoxidation, the Sharpless group created synthetic intermediates of various natural products: methymycin, erythromycin
, leukotriene
C-1, and (+)-disparlure.
As one of the few, highly enantioselective reactions during its time, many manipulations of the of the 2,3-epoxyalcohols have been developed.
The Sharpless Epoxidation has been used for the total synthesis of various carbohydrates, terpenes, leukotrienes, pheromones, and antibiotics.
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
to prepare 2,3-epoxyalcohols from primary and secondary allylic alcohol
Allyl alcohol
Allyl alcohol is an organic compound with the structural formula CH2=CHCH2OH. Like many alcohols,it is a water soluble, colourless liquid, but it is more toxic than typical small alcohols. Allyl alcohol is used as a raw material for the production of glycerol, but is used as a precursor to many...
s.

Diethyl tartrate
Diethyl tartrate is the ethyl ester of tartaric acid. It is chiral, showing both left- and right-handed forms, as well as a meso stereoisomer.In the Sharpless epoxidation, diethyl tartrate and titanium isopropoxide form a chiral catalyst in situ....
or diisopropyl tartrate
Diisopropyl tartrate
Diisopropyl tartrate is a diester of tartaric acid. It has a two chiral carbon atoms giving rise to three stereoisomeric variants. It is commonly used in asymmetric synthesis as a catalyst and as chiral building block for pharmaceuticals and agrochemicals...
) employed in the reaction. The oxidizing agent is tert-butyl hydroperoxide
Tert-Butyl hydroperoxide
tert-Butyl hydroperoxide is an organic peroxide widely used in a variety of oxidation processes, for example Sharpless epoxidation...
. Enantioselectivity is achieved by a catalyst formed from titanium tetra(isopropoxide) and diethyl tartrate. Only 5-10 mol% of the catalyst in the presence of 3Å
Ångström
The angstrom or ångström, is a unit of length equal to 1/10,000,000,000 of a meter . Its symbol is the Swedish letter Å....
molecular sieve
Molecular sieve
A molecular sieve is a material containing tiny pores of a precise and uniform size that is used as an adsorbent for gases and liquids.Molecules small enough to pass through the pores are adsorbed while larger molecules are not. It is different from a common filter in that it operates on a...
s (3Å MS) is necessary.
The Sharpless Epoxidation's success is due to five major reasons. First, epoxides can be easily converted into diol
Diol
A diol or glycol is a chemical compound containing two hydroxyl groups A geminal diol has two hydroxyl groups bonded to the same atom...
s, aminoalcohols or ether
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
s, so formation of chiral epoxides is a very important step in the synthesis of natural products. Second, the Sharpless Epoxidation reacts with many primary and secondary allylic alcohols. Third, the products of the Sharpless Epoxidation frequently have enantiomeric excess
Enantiomeric excess
The enantiomeric excess of a substance is a measure of how pure it is. In this case, the impurity is the undesired enantiomer .-Definition:...
es above 90%. Fourth, the products of the Sharpless Epoxidation are predictable using the Sharpless Epoxidation model. Finally, the reactants for the Sharpless Epoxidation are commercially available and relatively cheap.
Several reviews have been published.
K. Barry Sharpless
K. Barry Sharpless
Karl Barry Sharpless is an American chemist known for his work on stereoselective reactions.-Early years:Sharpless was born in Philadelphia. He graduated from Friends' Central School in 1959. He continued his studies at Dartmouth College and earned his Ph.D from Stanford University in 1968...
shared the 2001 Nobel prize in Chemistry
Nobel Prize in Chemistry
The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outstanding contributions in chemistry, physics, literature,...
for his work on asymmetric oxidations.
The prize was shared with William S. Knowles and Ryōji Noyori
Ryoji Noyori
is a Japanese chemist. He won the Nobel Prize in Chemistry in 2001. Noyori shared half of the prize with William S. Knowles for the study of chirally catalyzed hydrogenations; the second half of the Prize went to K. Barry Sharpless for his study in chirally catalyzed oxidation reactions...
.
Catalyst Structure
The structure of the catalyst is still uncertain. No studies have been conducted that definitively exclude other proposed catalysts. Regardless, all studies have concluded that the catalyst is a dimer of [Ti(tartrate)(OR)2] The putative catalyst was determined using X-ray structural determinations of model complexes which have the necessary structural components to catalyze the Sharpless Epoxidation.Selectivity
The chirality of the product of a Sharpless epoxidation can be predicted using the following mnemonic. Draw the double bond of interest lying flat. Draw a rectangle that around the double bond in the same plane as the carbons of the double bond. Towards the bottom right corner, draw the allylic alcohol. Place the other substituents in the appropriate corners. In this orientation, the (-) diester tartrate preferentially interacts with the top half of the molecule, and the (+) diester tartrate preferentially interacts with the bottom half of the molcule. This model seems to be valid despite substitution on the olefin. Selectivity decreases with larger R1, but increases with larger R2 and R3 (see introduction).The product of allylic 1,2-diols is incorrectly predicted by this model.
Kinetic Resolution
The Sharpless Epoxidation can also give kinetic resolutionKinetic resolution
In kinetic resolution, two enantiomers show different reaction rates in a chemical reaction, thereby creating an excess of the less reactive enantiomer. This excess goes through a maximum and disappears on full completion of the reaction. Kinetic resolution is a very old concept in organic...
of a racemic mixture of secondary 2,3-epoxyalcohols. While the yield of a kinetic resolution process cannot be higher than 50%, the enantiomeric excess
Enantiomeric excess
The enantiomeric excess of a substance is a measure of how pure it is. In this case, the impurity is the undesired enantiomer .-Definition:...
approaches 100% in some reactions.
Synthetic Utility
The Sharpless Epoxidation is viable with a large range of primary and secondary olefinic alcohols. Furthermore, with the exception noted above, a given dialkyl tartrate will preferentially add to the same face independent of the substitution on the olefin.To demonstrate the synthetic utility of the Sharpless Epoxidation, the Sharpless group created synthetic intermediates of various natural products: methymycin, erythromycin
Erythromycin
Erythromycin is a macrolide antibiotic that has an antimicrobial spectrum similar to or slightly wider than that of penicillin, and is often used for people who have an allergy to penicillins. For respiratory tract infections, it has better coverage of atypical organisms, including mycoplasma and...
, leukotriene
Leukotriene
Leukotrienes are fatty signaling molecules. They were first found in leukocytes . One of their roles is to trigger contractions in the smooth muscles lining the trachea; their overproduction is a major cause of inflammation in asthma and allergic rhinitis...
C-1, and (+)-disparlure.
As one of the few, highly enantioselective reactions during its time, many manipulations of the of the 2,3-epoxyalcohols have been developed.
The Sharpless Epoxidation has been used for the total synthesis of various carbohydrates, terpenes, leukotrienes, pheromones, and antibiotics.

