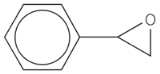
Styrene oxide
Encyclopedia
Styrene oxide is an epoxide
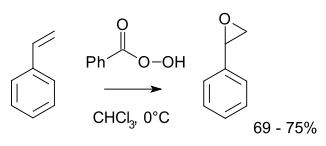
derived from styrene. It may be prepared by epoxidation of styrene with peroxybenzoic acid
, in the Prilezhaev reaction:
of styrene in humans or animals, resulting from oxidation by cytochrome P450. It is considered toxic, mutagenic, and possibly carcinogenic. Styrene oxide is subsequently hydrolyzed in vivo to styrene glycol by epoxide hydrolase
.
Styrene oxide has a chiral center and thus two enantiomers. It has been reported that the two enantiomers had different toxicokinetics
and toxicity. It was reported that the (R)-styrene oxide was preferentially formed in mice, especially in the lung, whereas the (S)-styrene oxide was preferentially generated in rats. In human volunteers, the cumulative excretion of the (S)-enantiomer of styrene glycol and mendelic acid were higher than the R form after exposure to styrene. In human liver microsomes, cytochrome P450-mediated styrene oxidation showed the production of more S enantiomer relative to the R enantiomer. It was also found that (S)-styrene oxide was preferentially hydrolyzed than the R enantiomer in human liver microsomes. Animal studies have shown that the (R)-enantiomer of styrene oxide was more toxic than the (S)-enantiomer in mice.
Epoxide
An epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in...
derived from styrene. It may be prepared by epoxidation of styrene with peroxybenzoic acid
Peroxybenzoic acid
Peroxybenzoic acid is a simple peroxy acid. It may be synthesized from benzoic acid and hydrogen peroxide, or by the treatment of benzoyl peroxide with sodium methoxide, followed by acidification....
, in the Prilezhaev reaction:

Toxicology
Styrene oxide is a main metaboliteMetabolite
Metabolites are the intermediates and products of metabolism. The term metabolite is usually restricted to small molecules. A primary metabolite is directly involved in normal growth, development, and reproduction. Alcohol is an example of a primary metabolite produced in large-scale by industrial...
of styrene in humans or animals, resulting from oxidation by cytochrome P450. It is considered toxic, mutagenic, and possibly carcinogenic. Styrene oxide is subsequently hydrolyzed in vivo to styrene glycol by epoxide hydrolase
Epoxide hydrolase
Epoxide hydrolase functions in detoxication during drug metabolism. It converts epoxides to trans-dihydrodiols, which can be conjugated and excreted from the body. Epoxides result from the degradation of aromatic compounds...
.
Styrene oxide has a chiral center and thus two enantiomers. It has been reported that the two enantiomers had different toxicokinetics
Toxicokinetics
Toxicokinetics is the description of what rate a chemical will enter the body and what happens to it once it is in the body. It is an application of pharmacokinetics to determine the relationship between the systemic exposure of a compound in experimental animals and its toxicity...
and toxicity. It was reported that the (R)-styrene oxide was preferentially formed in mice, especially in the lung, whereas the (S)-styrene oxide was preferentially generated in rats. In human volunteers, the cumulative excretion of the (S)-enantiomer of styrene glycol and mendelic acid were higher than the R form after exposure to styrene. In human liver microsomes, cytochrome P450-mediated styrene oxidation showed the production of more S enantiomer relative to the R enantiomer. It was also found that (S)-styrene oxide was preferentially hydrolyzed than the R enantiomer in human liver microsomes. Animal studies have shown that the (R)-enantiomer of styrene oxide was more toxic than the (S)-enantiomer in mice.

