
Dodecahedrane
Encyclopedia
Dodecahedrane is a chemical compound
(C20H20) first synthesised
by Leo Paquette
of Ohio State University
in 1982, primarily for the "aesthetically pleasing symmetry of the dodecahedral framework".
In this molecule
, each vertex is a carbon
atom that bonds to three neighbouring carbon atoms. The 108° angle of each regular pentagon is close to the ideal bond angle of 109.5° for an sp3 hybridised
atom. Each carbon atom is bonded to a hydrogen
atom as well. The molecule, like fullerene
, has Ih symmetry
, evidenced by its proton NMR
spectrum in which all hydrogen atoms appear at a single chemical shift
of 3.38 ppm. Dodecahedrane is one of the platonic hydrocarbons
, the others being cubane
and tetrahedrane
, and does not occur in nature.
of dodecahedrane. A review article published in 1978 just dealt with the different strategies that existed up to then. The first attempt was initiated in 1964 by R.B. Woodward
with the synthesis of the compound triquinacene which was thought to be able to simply dimerize to dodecahedrane. Other groups joined in the race, for example that of Philip Eaton
and Paul von Ragué Schleyer
Paquette
's 1982 organic synthesis
takes about 29 steps with raw materials cyclopentadiene
(2 equivalents 10 carbon atoms), dimethyl acetylenedicarboxylate
(4 carbon atoms) and allyltrimethylsilane (2 equivalents, 6 carbon atoms).
In the first leg of the procedure two molecules of cyclopentadiene
1 are coupled
together by reaction with elemental sodium
(forming the cyclopentadienyl complex
) and iodine
to dihydrofulvalene
2. Next up is a tandem Diels-Alder reaction
with dimethyl acetylenedicarboxylate
3 with desired sequence pentadiene-acetylene-pentadiene as in symmetrical adduct 4. An equal amount of asymmetric pentadiene-pentadiene-acetylene compound (4b) is formed and discarded.
In the next step of the sequence iodine is temporarily introduced via an iodolactonization of the diacid of 4 to dilactone 5. The ester
group is cleaved next by methanol
to the halohydrin
6, the alcohol
groups converted to ketone
groups in 7 by Jones oxidation
and the iodine groups reduced by a zinc-copper couple
in 8.
The final 6 carbon atoms are inserted in a nucleophilic addition
to the ketone groups of the carbanion
10 generated from allyltrimethylsilane 9 and n-butyllithium
. In the next step the vinyl silane 11 reacts with peracetic acid in acetic acid
in a radical substitution
to the dilactone 12 followed by an intramolecular
Friedel-Crafts alkylation with phosphorus pentoxide
to diketone 13. This molecule contains all required 20 carbon atoms and is also symmetrical which facilitates the construction of the remaining 5 carbon-carbon bond
s.
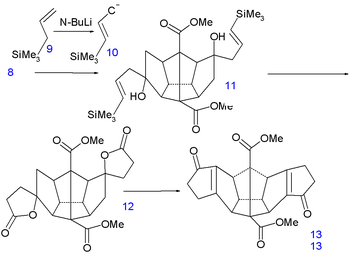
Reduction of the double bond
s in 13 to 14 is accomplished with hydrogenation
with palladium on carbon
and that of the ketone groups to alcohol groups in 15 by sodium borohydride
. Replacement of hydroxyl by chlorine
in 17 via nucleophilic aliphatic substitution takes place through the dilactone 16 (tosyl chloride). The first C-C bond forming reaction is a kind of Birch alkylation (lithium
, ammonia
) with the immediate reaction product trapped with chloromethyl phenyl ether, the other chlorine atom in 17 is simply reduced. This temporary appendix will in a later stage prevent unwanted enolization. The newly formed ketone
group then forms another C-C bond by photochemical Norrish reaction
to 19 whose alcohol group is induced to eliminate
with TsOH to alkene
20.
The double bond is reduced with hydrazine
and sequential diisobutylaluminum hydride reduction and pyridinium chlorochromate
oxidation of 21 forms the aldehyde
22. A second Norrish reaction then adds another C-C bond to alcohol 23 and having served its purpose the phenoxy tail is removed in several steps: a Birch reduction
to diol 24, oxidation with pyridinium chlorochromate
to ketoaldehyde 25 and a reverse Claisen condensation
to ketone 26. A third Norrish reaction produces alcohol 27 and a second dehydration
28 and another reduction 29 at which point the synthesis is left completely without functional group
s. The missing C-C bond is put in place by hydrogen pressurized dehydrogenation
with palladium on carbon
at 250°C to dodecahedrane 30.
.
ions (He+) at a film of C20H20, Cross, Saunders and Prinsbach managed to obtain a few micrograms of He@C20H20 — dodecahedrane with a helium atom trapped inside each molecule. This substance was described as quite stable, and the authors claimed to have produced the world's smallest helium balloons.
atoms yields the relatively unstable perfluorododecahedrane , which was obtained in milligram quantities by Wahl and others (2006). Trace amounts of the analogous perchlorododecahedrane were obtained, among other partially chlorinated derivatives, by reacting dissolved in liquid chlorine
under pressure at about 140 °C and under intense light for five days. Complete replacement by heavier halogen
s seems increasingly difficult due to their larger size. Half or more of the hydrogen atoms can be substituted by hydroxyl
groups to yield polyols, but the extreme compound remained elusive as of 2006.
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
(C20H20) first synthesised
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
by Leo Paquette
Leo Paquette
Leo Armand Paquette is an American organic chemist. He received his Ph.D. in 1959 from the Massachusetts Institute of Technology and has been a Professor of Chemistry at the Ohio State University since 1969. He is the author of over 1000 papers and has also edited works such as the Encyclopedia...
of Ohio State University
Ohio State University
The Ohio State University, commonly referred to as Ohio State, is a public research university located in Columbus, Ohio. It was originally founded in 1870 as a land-grant university and is currently the third largest university campus in the United States...
in 1982, primarily for the "aesthetically pleasing symmetry of the dodecahedral framework".
In this molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
, each vertex is a carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
atom that bonds to three neighbouring carbon atoms. The 108° angle of each regular pentagon is close to the ideal bond angle of 109.5° for an sp3 hybridised
Orbital hybridisation
In chemistry, hybridisation is the concept of mixing atomic orbitals to form new hybrid orbitals suitable for the qualitative description of atomic bonding properties. Hybridised orbitals are very useful in the explanation of the shape of molecular orbitals for molecules. It is an integral part...
atom. Each carbon atom is bonded to a hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
atom as well. The molecule, like fullerene
Fullerene
A fullerene is any molecule composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Spherical fullerenes are also called buckyballs, and they resemble the balls used in association football. Cylindrical ones are called carbon nanotubes or buckytubes...
, has Ih symmetry
Molecular symmetry
Molecular symmetry in chemistry describes the symmetry present in molecules and the classification of molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can predict or explain many of a molecule's chemical properties, such as its dipole moment...
, evidenced by its proton NMR
Proton NMR
Proton NMR is the application of nuclear magnetic resonance in NMR spectroscopy with respect to hydrogen-1 nuclei within the molecules of a substance, in order to determine the structure of its molecules. In samples where natural hydrogen is used, practically all of the hydrogen consists of the...
spectrum in which all hydrogen atoms appear at a single chemical shift
Chemical shift
In nuclear magnetic resonance spectroscopy, the chemical shift is the resonant frequency of a nucleus relative to a standard. Often the position and number of chemical shifts are diagnostic of the structure of a molecule...
of 3.38 ppm. Dodecahedrane is one of the platonic hydrocarbons
Platonic hydrocarbons
Platonic hydrocarbons are the molecular representation of platonic solid geometries with vertices replaced by carbon atoms and with edges replaced by chemical bonds...
, the others being cubane
Cubane
Cubane is a synthetic hydrocarbon molecule that consists of eight carbon atoms arranged at the corners of a cube, with one hydrogen atom attached to each carbon atom. A solid crystalline substance, cubane is one of the Platonic hydrocarbons. It was first synthesized in 1964 by Philip Eaton, a...
and tetrahedrane
Tetrahedrane
Tetrahedrane is a platonic hydrocarbon with chemical formula 44 and a tetrahedral structure. Extreme angle strain prevents this molecule from forming naturally....
, and does not occur in nature.
Total synthesis
For over 30 years several research groups have actively pursued the total synthesisTotal synthesis
In organic chemistry, a total synthesis is, in principle, the complete chemical synthesis of complex organic molecules from simpler pieces, usually without the aid of biological processes. In practice, these simpler pieces are commercially available in bulk and semi-bulk quantities, and are often...
of dodecahedrane. A review article published in 1978 just dealt with the different strategies that existed up to then. The first attempt was initiated in 1964 by R.B. Woodward
Robert Burns Woodward
Robert Burns Woodward was an American organic chemist, considered by many to be the preeminent organic chemist of the twentieth century...
with the synthesis of the compound triquinacene which was thought to be able to simply dimerize to dodecahedrane. Other groups joined in the race, for example that of Philip Eaton
Philip Eaton
Philip E. Eaton is a Professor Emeritus of Chemistry at the University of Chicago. He and his fellow researchers were the first to synthesize the "impossible" cubane molecule in 1964....
and Paul von Ragué Schleyer
Paul von Rague Schleyer
Paul von Ragué Schleyer is an organic physical chemist of substantial significance whose research has been cited with great frequency. A 1997 survey indicated that Dr. Schleyer was, at the time, the world's third most cited chemist, with over 1100 technical papers produced...
Paquette
Leo Paquette
Leo Armand Paquette is an American organic chemist. He received his Ph.D. in 1959 from the Massachusetts Institute of Technology and has been a Professor of Chemistry at the Ohio State University since 1969. He is the author of over 1000 papers and has also edited works such as the Encyclopedia...
's 1982 organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
takes about 29 steps with raw materials cyclopentadiene
Cyclopentadiene
Cyclopentadiene is an organic compound with the formula C5H6. This colorless liquid has a strong and unpleasant odor. At room temperature, this cyclic diene dimerizes over the course of hours to give dicyclopentadiene via a Diels–Alder reaction...
(2 equivalents 10 carbon atoms), dimethyl acetylenedicarboxylate
Dimethyl acetylenedicarboxylate
Dimethyl acetylenedicarboxylate is the organic compound with the formula CH3O2CC2CO2CH3. This ester, which exists as a liquid at room temperature, is highly electrophilic. As such, DMAD, as it is commonly called in the laboratory, is widely employed as a dienophile in cycloaddition reactions,...
(4 carbon atoms) and allyltrimethylsilane (2 equivalents, 6 carbon atoms).
In the first leg of the procedure two molecules of cyclopentadiene
Cyclopentadiene
Cyclopentadiene is an organic compound with the formula C5H6. This colorless liquid has a strong and unpleasant odor. At room temperature, this cyclic diene dimerizes over the course of hours to give dicyclopentadiene via a Diels–Alder reaction...
1 are coupled
Coupling reaction
A coupling reaction in organic chemistry is a catch-all term for a variety of reactions where two hydrocarbon fragments are coupled with the aid of a metal catalyst...
together by reaction with elemental sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
(forming the cyclopentadienyl complex
Cyclopentadienyl complex
A cyclopentadienyl complex is a metal complex with one or more cyclopentadienyl groups . Based on the type of bonding between the metals and the cyclopentadienyl]] moieties, cyclopentadienyl complexes are classified into the following three categories: a) π-complexes, b) σ-complexes, and c) ionic...
) and iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
to dihydrofulvalene
Fulvalene
A fulvalene is a hydrocarbon obtained by formally cross-conjugating two rings through a common exocyclic double bond. The name is derived from the similarly structured fulvenes which lack one ring...
2. Next up is a tandem Diels-Alder reaction
Diels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
with dimethyl acetylenedicarboxylate
Dimethyl acetylenedicarboxylate
Dimethyl acetylenedicarboxylate is the organic compound with the formula CH3O2CC2CO2CH3. This ester, which exists as a liquid at room temperature, is highly electrophilic. As such, DMAD, as it is commonly called in the laboratory, is widely employed as a dienophile in cycloaddition reactions,...
3 with desired sequence pentadiene-acetylene-pentadiene as in symmetrical adduct 4. An equal amount of asymmetric pentadiene-pentadiene-acetylene compound (4b) is formed and discarded.
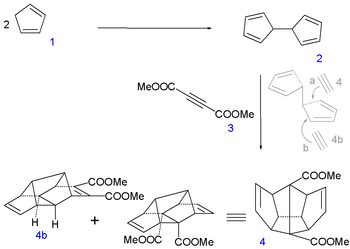 |
|valign=top |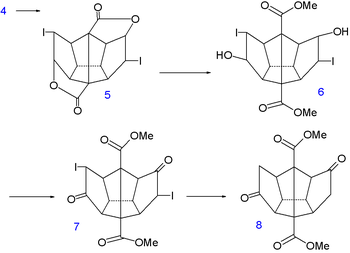 |
|
| Dodecahedrane synthesis part I | Dodecahedrane synthesis part II | |
In the next step of the sequence iodine is temporarily introduced via an iodolactonization of the diacid of 4 to dilactone 5. The ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
group is cleaved next by methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
to the halohydrin
Halohydrin
A halohydrin or a haloalcohol is a type of organic compound or functional group in which one carbon atom has a halogen substituent, and an adjacent carbon atom has a hydroxyl substituent. They are derived from alcohols are therefore characterized by the presence of both the hydroxyl functional...
6, the alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
groups converted to ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
groups in 7 by Jones oxidation
Jones oxidation
The Jones oxidation, is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones, respectively. It is named after its discoverer, Sir Ewart Jones....
and the iodine groups reduced by a zinc-copper couple
Zinc-copper couple
Zinc-copper couple is an alloy of zinc and copper that is employed as a reagent in organic synthesis. The “couple” was popularized after the report by Simmons and Smith in 1959 of its application as an activated source of zinc required for formation of an organozinc reagent in the Simmons-Smith...
in 8.
 |
|valign=top |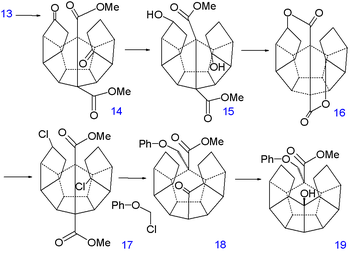 |
|
| Dodecahedrane synthesis part III | Dodecahedrane synthesis part IV | |
The final 6 carbon atoms are inserted in a nucleophilic addition
Nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile....
to the ketone groups of the carbanion
Carbanion
A carbanion is an anion in which carbon has an unshared pair of electrons and bears a negative charge usually with three substituents for a total of eight valence electrons. The carbanion exists in a trigonal pyramidal geometry. Formally a carbanion is the conjugate base of a carbon acid.where B...
10 generated from allyltrimethylsilane 9 and n-butyllithium
N-Butyllithium
n-Butyllithium is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene...
. In the next step the vinyl silane 11 reacts with peracetic acid in acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
in a radical substitution
Radical substitution
In organic chemistry, a radical substitution reaction is a substitution reaction involving free radicals as a reactive intermediate.The reaction always involves at least two steps, and possibly a third....
to the dilactone 12 followed by an intramolecular
Intramolecular
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.- Examples :...
Friedel-Crafts alkylation with phosphorus pentoxide
Phosphorus pentoxide
Phosphorus pentoxide is a chemical compound with molecular formula P4O10 . This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant.-Structure:...
to diketone 13. This molecule contains all required 20 carbon atoms and is also symmetrical which facilitates the construction of the remaining 5 carbon-carbon bond
Carbon-carbon bond
A carbon–carbon bond is a covalent bond between two carbon atoms. The most common form is the single bond: a bond composed of two electrons, one from each of the two atoms. The carbon–carbon single bond is a sigma bond and is said to be formed between one hybridized orbital from each...
s.
Reduction of the double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
s in 13 to 14 is accomplished with hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
with palladium on carbon
Palladium on carbon
Palladium on carbon, often referred to as Pd/C, is a form of palladium used for catalysis. It is usually used for catalytic hydrogenations in organic chemistry...
and that of the ketone groups to alcohol groups in 15 by sodium borohydride
Sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate, is an inorganic compound with the formula NaBH4. This white solid, usually encountered as a powder, is a versatile reducing agent that finds wide application in chemistry, both in the laboratory and on a technical scale. Large amounts are...
. Replacement of hydroxyl by chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
in 17 via nucleophilic aliphatic substitution takes place through the dilactone 16 (tosyl chloride). The first C-C bond forming reaction is a kind of Birch alkylation (lithium
Lithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
, ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
) with the immediate reaction product trapped with chloromethyl phenyl ether, the other chlorine atom in 17 is simply reduced. This temporary appendix will in a later stage prevent unwanted enolization. The newly formed ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
group then forms another C-C bond by photochemical Norrish reaction
Norrish reaction
The Norrish reaction in organic chemistry describes the photochemical reactions taking place with ketones and aldehydes. This type of reaction is subdivided in Norrish type I reactions and Norrish type II reactions...
to 19 whose alcohol group is induced to eliminate
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
with TsOH to alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
20.
 |
|valign=top |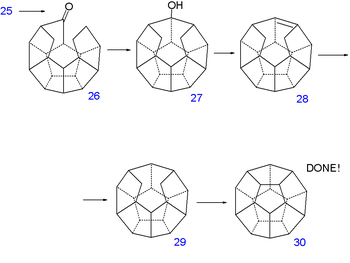 |
|
| Dodecahedrane synthesis part V | Dodecahedrane synthesis part VI | |
The double bond is reduced with hydrazine
Hydrazine
Hydrazine is an inorganic compound with the formula N2H4. It is a colourless flammable liquid with an ammonia-like odor. Hydrazine is highly toxic and dangerously unstable unless handled in solution. Approximately 260,000 tons are manufactured annually...
and sequential diisobutylaluminum hydride reduction and pyridinium chlorochromate
Pyridinium chlorochromate
Pyridinium chlorochromate is a reddish orange solid reagent used to oxidize primary alcohols to aldehydes and secondary alcohols to ketones. Pyridinium chlorochromate, or PCC, will not fully oxidize a primary alcohol to the carboxylic acid as does the Jones reagent. A disadvantage to using PCC is...
oxidation of 21 forms the aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
22. A second Norrish reaction then adds another C-C bond to alcohol 23 and having served its purpose the phenoxy tail is removed in several steps: a Birch reduction
Birch reduction
The Birch Reduction is an organic reaction which is particularly useful in synthetic organic chemistry. The reaction was reported in 1944 by the Australian chemist Arthur Birch working in the Dyson Perrins Laboratory in the University of Oxford, building on earlier work by Wooster and Godfrey in...
to diol 24, oxidation with pyridinium chlorochromate
Pyridinium chlorochromate
Pyridinium chlorochromate is a reddish orange solid reagent used to oxidize primary alcohols to aldehydes and secondary alcohols to ketones. Pyridinium chlorochromate, or PCC, will not fully oxidize a primary alcohol to the carboxylic acid as does the Jones reagent. A disadvantage to using PCC is...
to ketoaldehyde 25 and a reverse Claisen condensation
Claisen condensation
The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base, resulting in a β-keto ester or a β-diketone...
to ketone 26. A third Norrish reaction produces alcohol 27 and a second dehydration
Dehydration
In physiology and medicine, dehydration is defined as the excessive loss of body fluid. It is literally the removal of water from an object; however, in physiological terms, it entails a deficiency of fluid within an organism...
28 and another reduction 29 at which point the synthesis is left completely without functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
s. The missing C-C bond is put in place by hydrogen pressurized dehydrogenation
Dehydrogenation
Dehydrogenation is a chemical reaction that involves the elimination of hydrogen . It is the reverse process of hydrogenation. Dehydrogenation reactions may be either large scale industrial processes or smaller scale laboratory procedures....
with palladium on carbon
Palladium on carbon
Palladium on carbon, often referred to as Pd/C, is a form of palladium used for catalysis. It is usually used for catalytic hydrogenations in organic chemistry...
at 250°C to dodecahedrane 30.
Synthesis from pagodane
In 1987 an alternative synthesis route was found by W.-D. Fessner and others, through the isomer pagodanePagodane
Pagodane is an organic compound with formula whose carbon skeleton was said to resemble a pagoda, hence the name. It is a polycyclic hydrocarbon whose molecule has the D2h point symmetry group. The compound is a highly crystalline solid that melts at 243 °C, is barely soluble in most organic...
.
The world's smallest helium balloons
By shooting heliumHelium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
ions (He+) at a film of C20H20, Cross, Saunders and Prinsbach managed to obtain a few micrograms of He@C20H20 — dodecahedrane with a helium atom trapped inside each molecule. This substance was described as quite stable, and the authors claimed to have produced the world's smallest helium balloons.
Derivatives
A variety of dodecahedrane derivatives have been synthesized and reported in the literature. Substitution of all 20 hydrogens by fluorineFluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
atoms yields the relatively unstable perfluorododecahedrane , which was obtained in milligram quantities by Wahl and others (2006). Trace amounts of the analogous perchlorododecahedrane were obtained, among other partially chlorinated derivatives, by reacting dissolved in liquid chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
under pressure at about 140 °C and under intense light for five days. Complete replacement by heavier halogen
Halogen
The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
s seems increasingly difficult due to their larger size. Half or more of the hydrogen atoms can be substituted by hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
groups to yield polyols, but the extreme compound remained elusive as of 2006.

