Jones oxidation
Encyclopedia
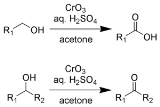
The Jones oxidation, is an organic reaction
for the oxidation
of primary and secondary alcohol
s to carboxylic acid
s and ketone
s, respectively. It is named after its discoverer, Sir Ewart Jones
.
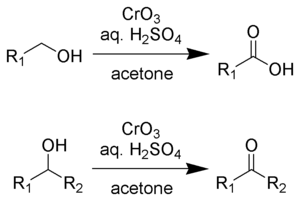 Jones reagent is a solution of chromium trioxide
Jones reagent is a solution of chromium trioxide
in dilute sulfuric acid
and acetone. A mixture of potassium dichromate and dilute sulfuric acid can also be used. The solvent acetone
markedly affects the properties of the chromic acid. The oxidation is very rapid, quite exothermic
, and the yields are typically high. The reagent rarely oxidizes unsaturated bonds.
For oxidation of primary alcohols to carboxylic acids, one equivalent of Jones reagent is required for each substrate. The aldehyde is an intermediate.
The inorganic products are green, characteristic of chromium(III) aquo complexes
.
: These esters have the formula CrO3(OCH2R)–
Like conventional esters, the formation of this chromate ester
is accelerated by the acid. These esters can be isolated when the alcohol lacks α-C-H bonds. For example, using tert-butanol
, one can isolate ((CH3)3CO)2CrO2 (which is a good oxidant). The chromate esters degrade, releasing the carbonyl product and an ill-defined Cr(IV) product:
The partially deuterated alcohols HOCD2R oxidize about six times slower than the undeuterated derivatives. This large kinetic isotope effect
shows that the C–H (or C-D) bond breaks in the rate-determining step. The reaction stoichiometry implicates the Cr(IV) species "CrO2OH–", which comproportionates with the chromic acid to give a Cr(V) oxide, which also functions as an oxidant for the alcohol.
The oxidation of the aldehydes is proposed to proceed via the formation of hemiacetal
-like intermediates, which arise from the addition of the O3CrO-H- bond across the C=O bond.
. A variety of spectroscopic techniques, including IR
can be used to monitor the progress of a Jones Oxidation reaction and confirm the presence of the oxidized product. At one time the Jones oxidation was used in primitive breathalyzer
s. Aminoindans, which are of pharmalogical interest, are prepared by the oxidation of the alcohol to ketone which is converted into an amino group. The alcohol is oxidized to the ketone with the Jones reagent. The reagent was once used to prepare salicylic acid
, a precursor to aspirin. Methcathinone is a psychoactive stimulant that is sometimes used as an addictive recreational drug
. It can be oxidized from certain alcohols using the Jones reagent.
and pyridinium chlorochromate
The Sarett oxidation
is a similar process.
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
for the oxidation
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
of primary and secondary alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s to carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
s and ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s, respectively. It is named after its discoverer, Sir Ewart Jones
Ewart Jones
Professor Sir Ewart Ray Herbert Jones was a Welsh organic chemist and academic administrator, whose fields of expertise led him to discoveries into the chemistry of natural products, mainly steroids, terpenes and vitamins...
.

Chromium trioxide
Chromium trioxide is the inorganic compound with the formula CrO3. It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name.This compound is a dark-red/orange brown solid, which dissolves in water concomitant with hydrolysis...
in dilute sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
and acetone. A mixture of potassium dichromate and dilute sulfuric acid can also be used. The solvent acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
markedly affects the properties of the chromic acid. The oxidation is very rapid, quite exothermic
Exothermic
In thermodynamics, the term exothermic describes a process or reaction that releases energy from the system, usually in the form of heat, but also in the form of light , electricity , or sound...
, and the yields are typically high. The reagent rarely oxidizes unsaturated bonds.
Stoichiometry
Jones reagent will convert primary and secondary alcohols to aldehydes and ketones, respectively. Depending on the reaction conditions, the aldehydes may then be converted to carboxylic acids. For oxidations to the aldehydes and ketones, two equivalents of chromic acid oxidize three equivalents of the alcohol:- 2 HCrO4– + 3 RR'C(OH)H + 8 H+ + 4 H2O → 2 [Cr(H2O)6]3+ + 3 RR'CO
For oxidation of primary alcohols to carboxylic acids, one equivalent of Jones reagent is required for each substrate. The aldehyde is an intermediate.
- 4 HCrO4– + 3 RCH2OH + 16 H+ + 11 H2O → 4 [Cr(H2O)6]3+ + 3 RCOOH
The inorganic products are green, characteristic of chromium(III) aquo complexes
Metal aquo complex
Metal aquo complexes are coordination compounds containing metal ions with only water as a ligand. These complexes are the predominant species in aqueous solutions of many metal salts, such as metal nitrates, sulfates, and perchlorates. They have the general stoichiometry [Mn]z+. Their behavior...
.
Mechanism
Like many other oxidations of alcohols by metal oxides, the reaction proceeds via the formation of a mixed esterEster
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
: These esters have the formula CrO3(OCH2R)–
- CrO3(OH)– + RCH2OH → CrO3(OCH2R)– + H2O
Like conventional esters, the formation of this chromate ester
Chromate ester
A chromate ester is a chemical structure that contains a chromium atom in a +6 oxidation state that is connected via an oxygen linkage to a carbon atom. The Cr itself is in its chromate form, with several oxygens attached, and the Cr–O–C attachment makes this chemical group structurally similar to...
is accelerated by the acid. These esters can be isolated when the alcohol lacks α-C-H bonds. For example, using tert-butanol
Tert-Butanol
tert-Butanol, or 2-methyl-2-propanol, is the simplest tertiary alcohol. It is one of the four isomers of butanol. tert-Butanol is a clear liquid with a camphor-like odor. It is very soluble in water and miscible with ethanol and diethyl ether...
, one can isolate ((CH3)3CO)2CrO2 (which is a good oxidant). The chromate esters degrade, releasing the carbonyl product and an ill-defined Cr(IV) product:
- CrO3(OCH2R)– → "CrO2OH–" + O=CHR
The partially deuterated alcohols HOCD2R oxidize about six times slower than the undeuterated derivatives. This large kinetic isotope effect
Kinetic isotope effect
The kinetic isotope effect is the ratio of reaction rates of two different isotopically labeled molecules in a chemical reaction. It is also called "isotope fractionation," although this term is somewhat broader in meaning...
shows that the C–H (or C-D) bond breaks in the rate-determining step. The reaction stoichiometry implicates the Cr(IV) species "CrO2OH–", which comproportionates with the chromic acid to give a Cr(V) oxide, which also functions as an oxidant for the alcohol.
The oxidation of the aldehydes is proposed to proceed via the formation of hemiacetal
Hemiacetal
Hemiacetals and hemiketals are compounds that are derived from aldehydes and ketones respectively. The Greek word hèmi means half...
-like intermediates, which arise from the addition of the O3CrO-H- bond across the C=O bond.
Illustrative reactions and applications
Although useful reagent for some applications, due to the carcinogenic nature of chromium(VI), the Jones oxidation has slowly been replaced by other oxidation methods. It remains useful in organic synthesisOrganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
. A variety of spectroscopic techniques, including IR
Infrared spectroscopy
Infrared spectroscopy is the spectroscopy that deals with the infrared region of the electromagnetic spectrum, that is light with a longer wavelength and lower frequency than visible light. It covers a range of techniques, mostly based on absorption spectroscopy. As with all spectroscopic...
can be used to monitor the progress of a Jones Oxidation reaction and confirm the presence of the oxidized product. At one time the Jones oxidation was used in primitive breathalyzer
Breathalyzer
A breathalyzer or breathalyser is a device for estimating blood alcohol content from a breath sample...
s. Aminoindans, which are of pharmalogical interest, are prepared by the oxidation of the alcohol to ketone which is converted into an amino group. The alcohol is oxidized to the ketone with the Jones reagent. The reagent was once used to prepare salicylic acid
Salicylic acid
Salicylic acid is a monohydroxybenzoic acid, a type of phenolic acid and a beta hydroxy acid. This colorless crystalline organic acid is widely used in organic synthesis and functions as a plant hormone. It is derived from the metabolism of salicin...
, a precursor to aspirin. Methcathinone is a psychoactive stimulant that is sometimes used as an addictive recreational drug
Drug
A drug, broadly speaking, is any substance that, when absorbed into the body of a living organism, alters normal bodily function. There is no single, precise definition, as there are different meanings in drug control law, government regulations, medicine, and colloquial usage.In pharmacology, a...
. It can be oxidized from certain alcohols using the Jones reagent.
Related reagents
Several chromium oxides are used for related oxidations. These include Collins reagentCollins reagent
Collins reagent is the complex of chromium oxide with pyridine in dichloromethane. It is used to selectively oxidize primary alcohols to the aldehyde, and will tolerate many other functional groups within the molecule....
and pyridinium chlorochromate
Pyridinium chlorochromate
Pyridinium chlorochromate is a reddish orange solid reagent used to oxidize primary alcohols to aldehydes and secondary alcohols to ketones. Pyridinium chlorochromate, or PCC, will not fully oxidize a primary alcohol to the carboxylic acid as does the Jones reagent. A disadvantage to using PCC is...
The Sarett oxidation
Sarett oxidation
The Sarett oxidation is the oxidation of an alcohol to a ketone or an aldehyde using chromium trioxide and pyridine. Primary alcohols will only be oxidised to the aldehyde and not on to carboxylic acids....
is a similar process.

