Tetrahedrane
Encyclopedia
Tetrahedrane is a platonic hydrocarbon with chemical formula
44 and a tetrahedral
structure. Extreme angle strain
(carbon bond angles deviate considerably from the tetrahedral bond angle of 109.5°) prevents this molecule from forming naturally.
In 1978, Günther Maier prepared a stable tetrahedrane with four tert-butyl substituent
s. These substituents are very bulky, and completely envelop the tetrahedrane core. Maier suggested that bonds in the core are prevented from breaking because this would force the substituents closer together (corset effect) resulting in Van der Waals strain
. Tetrahedrane is one of the possible platonic hydrocarbons
and has the IUPAC name tricyclo[1.1.0.02,4]butane.
of an alkyne
with t-Bu substituted maleic anhydride
, followed by rearrangement with carbon dioxide expulsion to a cyclopentadienone and its bromination, followed by addition of the fourth t-Bu group and a photochemical rearrangement with expulsion of carbon monoxide
.
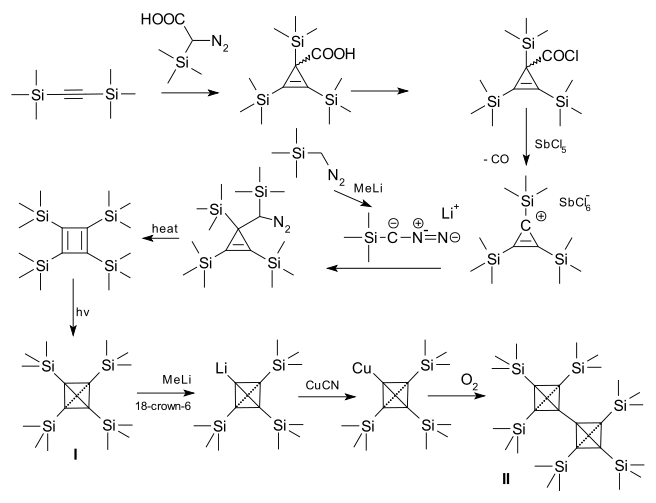
 In tetra(trimethylsilyl)tetrahedrane (I) the tert-butyl groups have been replaced by trimethylsilyl
In tetra(trimethylsilyl)tetrahedrane (I) the tert-butyl groups have been replaced by trimethylsilyl
groups. This compound is far more stable than the tert-butyl analogue. The silicon-carbon bond
is longer than a carbon-carbon bond, and therefore the corset effect is reduced. On the other hand, the trimethylsilyl group is a sigma donor
which explains the increased stabilization of the tetrahedrane. Whereas the tert-butyl tetrahedrane melts at 135 °C, at which temperature decomposition to the cyclobutadiene starts, the trimethyl silyl tetrahedrane melts at a much higher temperature of 202 °C, and is stable up to 300 °C, at which point it reverts to the acetylene
starting material.
The tetrahedrane skeleton is made up of banana bond
s, and hence the carbon atoms are high in s-orbital character. From NMR
, sp hybridization can be deduced, normally reserved for triple bond
s. As a consequence the bond length
s are unusually short with 152 picometers. The latest development is the organic synthesis
and characterization of the tetrahedrane dimer (II). The connecting bond is even shorter with 143.6 pm. An ordinary carbon carbon bond has a length of 154 pm.
. The standard silicon silicon bond is much longer (235 pm) and the cage is again enveloped by a total of 16 trimethylsilyl
groups. This makes the compound thermally stable. The silatetrahedrane can be reduced with potassium graphite to the tetrasilatetrahedranide potassium salt. In this compound one of the silicon atoms of the cage has lost a silyl substituent and carries a negative charge. The potassium cation can be captured by a crown ether
and in the resulting complex potassium and the silyl anion are separated by a distance of 885 pm. One of the Si- - Si bonds is now 272 pm and its silicon atom has an inverted tetrahedral geometry. Furthermore the four cage silicon atoms are equivalent on the NMR
timescale due to migrations of the silyl substituents over the cage.
The dimerization reaction observed for the carbon tetrahedrane compound is also attempted for a tetrasilatetrahedrane. In this tetrahedrane the cage is protected by 4 so-called super silyl groups in which a silicon atom has 3 tert-butyl substituents. The dimer does not materialize but a reaction with iodine
in benzene followed by reaction with the tri-tert-butyl sila anion results in the formation of an eight membered silicon cluster compound which can be described as a Si2 dumbbell (length 229 picometer and with inversion of tetrahedral geometry) sandwiched between two almost parallel Si3 rings.
In known eight-membered clusters of in the same carbon group
, tin
Sn8R6 and germanium
Ge8R6 the cluster atoms are located on the corners of a cube.
Locking away a tetrahedrane molecule inside a fullerene has only been attempted in silico
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
44 and a tetrahedral
Tetrahedron
In geometry, a tetrahedron is a polyhedron composed of four triangular faces, three of which meet at each vertex. A regular tetrahedron is one in which the four triangles are regular, or "equilateral", and is one of the Platonic solids...
structure. Extreme angle strain
Angle strain
Angle strain, also called Baeyer strain in cyclic molecules, is the resistance associated with bond angle compression or bond angle expansion. It occurs when bond angles deviate from the ideal bond angles to achieve maximum bond strength in a specific chemical conformation...
(carbon bond angles deviate considerably from the tetrahedral bond angle of 109.5°) prevents this molecule from forming naturally.
In 1978, Günther Maier prepared a stable tetrahedrane with four tert-butyl substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
s. These substituents are very bulky, and completely envelop the tetrahedrane core. Maier suggested that bonds in the core are prevented from breaking because this would force the substituents closer together (corset effect) resulting in Van der Waals strain
Van der Waals strain
In chemistry, van der Waals strain is strain resulting from van der Waals repulsion when two substituents in a molecule approach each other with a distance less than the sum of their van der Waals radii. Van der Waals strain is also called van der Waals repulsion and is related to steric hindrance...
. Tetrahedrane is one of the possible platonic hydrocarbons
Platonic hydrocarbons
Platonic hydrocarbons are the molecular representation of platonic solid geometries with vertices replaced by carbon atoms and with edges replaced by chemical bonds...
and has the IUPAC name tricyclo[1.1.0.02,4]butane.
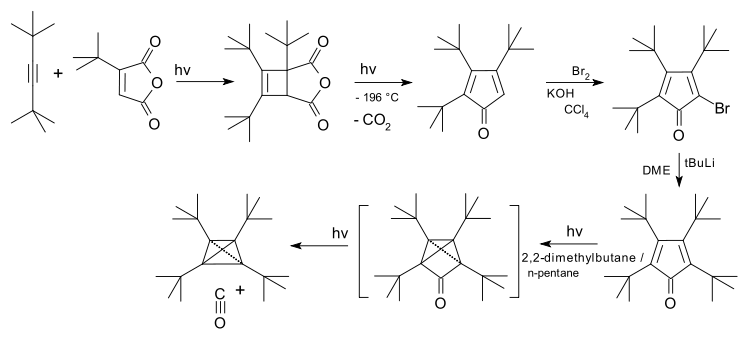
Tetra-tert-butyltetrahedrane
The tert-butyl substituted compound was first synthesised starting from a cycloadditionCycloaddition
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
of an alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
with t-Bu substituted maleic anhydride
Maleic anhydride
Maleic anhydride is an organic compound with the formula C2H22O. It is the acid anhydride of maleic acid and in its pure state it is a colourless or white solid with an acrid odour....
, followed by rearrangement with carbon dioxide expulsion to a cyclopentadienone and its bromination, followed by addition of the fourth t-Bu group and a photochemical rearrangement with expulsion of carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
.
Tetra(trimethylsilyl)tetrahedrane

Trimethylsilyl
A trimethylsilyl group is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom [−Si3], which is in turn bonded to the rest of a molecule...
groups. This compound is far more stable than the tert-butyl analogue. The silicon-carbon bond
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
is longer than a carbon-carbon bond, and therefore the corset effect is reduced. On the other hand, the trimethylsilyl group is a sigma donor
Sigma bond
In chemistry, sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most clearly defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a σ-bond is...
which explains the increased stabilization of the tetrahedrane. Whereas the tert-butyl tetrahedrane melts at 135 °C, at which temperature decomposition to the cyclobutadiene starts, the trimethyl silyl tetrahedrane melts at a much higher temperature of 202 °C, and is stable up to 300 °C, at which point it reverts to the acetylene
Acetylene
Acetylene is the chemical compound with the formula C2H2. It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in pure form and thus is usually handled as a solution.As an alkyne, acetylene is unsaturated because...
starting material.
The tetrahedrane skeleton is made up of banana bond
Banana bond
Bent bond, also known as banana bond, is a term in organic chemistry that refers to a type of covalent chemical bond with a geometry somewhat reminiscent of a banana...
s, and hence the carbon atoms are high in s-orbital character. From NMR
NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy, is a research technique that exploits the magnetic properties of certain atomic nuclei to determine physical and chemical properties of atoms or the molecules in which they are contained...
, sp hybridization can be deduced, normally reserved for triple bond
Triple bond
A triple bond in chemistry is a chemical bond between two chemical elements involving six bonding electrons instead of the usual two in a covalent single bond. The most common triple bond, that between two carbon atoms, can be found in alkynes. Other functional groups containing a triple bond are...
s. As a consequence the bond length
Bond length
- Explanation :Bond length is related to bond order, when more electrons participate in bond formation the bond will get shorter. Bond length is also inversely related to bond strength and the bond dissociation energy, as a stronger bond will be shorter...
s are unusually short with 152 picometers. The latest development is the organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
and characterization of the tetrahedrane dimer (II). The connecting bond is even shorter with 143.6 pm. An ordinary carbon carbon bond has a length of 154 pm.
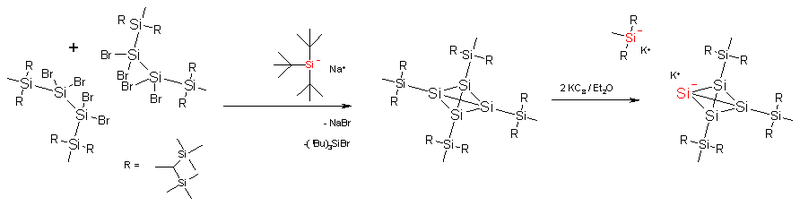
Tetrasilatetrahedrane
In tetrasilatetrahedrane the carbon atoms in the tetrahedrane cage are replaced by siliconSilicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
. The standard silicon silicon bond is much longer (235 pm) and the cage is again enveloped by a total of 16 trimethylsilyl
Trimethylsilyl
A trimethylsilyl group is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom [−Si3], which is in turn bonded to the rest of a molecule...
groups. This makes the compound thermally stable. The silatetrahedrane can be reduced with potassium graphite to the tetrasilatetrahedranide potassium salt. In this compound one of the silicon atoms of the cage has lost a silyl substituent and carries a negative charge. The potassium cation can be captured by a crown ether
Crown ether
Crown ethers are cyclic chemical compounds that consist of a ring containing several ether groups. The most common crown ethers are oligomers of ethylene oxide, the repeating unit being ethyleneoxy, i.e., -CH2CH2O-. Important members of this series are the tetramer , the pentamer , and the hexamer...
and in the resulting complex potassium and the silyl anion are separated by a distance of 885 pm. One of the Si- - Si bonds is now 272 pm and its silicon atom has an inverted tetrahedral geometry. Furthermore the four cage silicon atoms are equivalent on the NMR
NMR
NMR may refer to:Applications of Nuclear Magnetic Resonance:* Nuclear magnetic resonance* NMR spectroscopy* Solid-state nuclear magnetic resonance* Protein nuclear magnetic resonance spectroscopy* Proton NMR* Carbon-13 NMR...
timescale due to migrations of the silyl substituents over the cage.
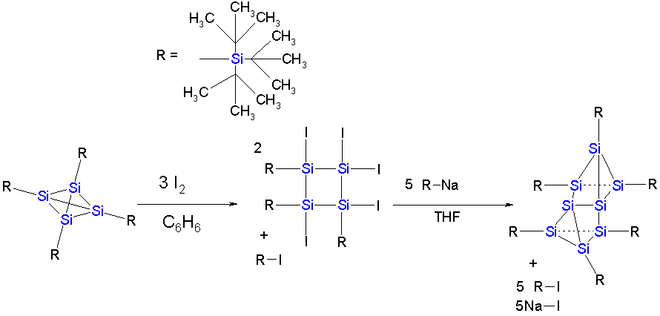
The dimerization reaction observed for the carbon tetrahedrane compound is also attempted for a tetrasilatetrahedrane. In this tetrahedrane the cage is protected by 4 so-called super silyl groups in which a silicon atom has 3 tert-butyl substituents. The dimer does not materialize but a reaction with iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
in benzene followed by reaction with the tri-tert-butyl sila anion results in the formation of an eight membered silicon cluster compound which can be described as a Si2 dumbbell (length 229 picometer and with inversion of tetrahedral geometry) sandwiched between two almost parallel Si3 rings.
In known eight-membered clusters of in the same carbon group
Carbon group
The carbon group is a periodic table group consisting of carbon , silicon , germanium , tin , lead , and ununquadium ....
, tin
Tin
Tin is a chemical element with the symbol Sn and atomic number 50. It is a main group metal in group 14 of the periodic table. Tin shows chemical similarity to both neighboring group 14 elements, germanium and lead and has two possible oxidation states, +2 and the slightly more stable +4...
Sn8R6 and germanium
Germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is a lustrous, hard, grayish-white metalloid in the carbon group, chemically similar to its group neighbors tin and silicon. The isolated element is a semiconductor, with an appearance most similar to elemental silicon....
Ge8R6 the cluster atoms are located on the corners of a cube.
Tetranitrotetrahedrane
Due to its bond strain and perfect oxygen balance, tetranitrotetrahedrane, an analogue of tetrahedrane with four nitro group substituents, has potential as a high-performance energetic material. The addition of these nitro groups is likely to reduce tetranitrohedrane's stability.Tetrahedrane outlook
Unsubstituted tetrahedrane is an as yet elusive molecule but it is predicted to be kinetically stable. One strategy that has been explored (but thus far failed) is reaction of propene with atomic carbonAtomic carbon
Atomic carbon in chemistry is single carbon atom with chemical formula :C: - in effect a dicarbene.This very short lived species is created by passing a large current through two adjacent carbon rods, generating an electric arc. Atomic carbon is generated in the process...
Locking away a tetrahedrane molecule inside a fullerene has only been attempted in silico
In silico
In silico is an expression used to mean "performed on computer or via computer simulation." The phrase was coined in 1989 as an analogy to the Latin phrases in vivo and in vitro which are commonly used in biology and refer to experiments done in living organisms and outside of living organisms,...