
Allotropes of carbon
Encyclopedia
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
.
Diamond
Diamond is one of the most well known allotropesAllotropy
Allotropy or allotropism is the property of some chemical elements to exist in two or more different forms, known as allotropes of these elements...
of carbon. The hardness and high dispersion of light of diamond make it useful for both industrial applications and jewellery. Diamond is the hardest known natural mineral
Mineral
A mineral is a naturally occurring solid chemical substance formed through biogeochemical processes, having characteristic chemical composition, highly ordered atomic structure, and specific physical properties. By comparison, a rock is an aggregate of minerals and/or mineraloids and does not...
. This makes it an excellent abrasive and makes it hold polish and luster extremely well. No known naturally occurring substance can cut (or even scratch) a diamond, except another diamond.
The market for industrial-grade diamonds operates much differently from its gem-grade counterpart. Industrial diamonds are valued mostly for their hardness and heat conductivity, making many of the gemological
Gemology
Gemology or gemmology is the science dealing with natural and artificial gems and gemstones. It is considered a geoscience and a branch of mineralogy...
characteristics of diamond, including clarity and color, mostly irrelevant. This helps explain why 80% of mined diamonds (equal to about 100 million carats or 20 tonnes annually) are unsuitable for use as gemstones and known as bort
Bort
Bort or boart is a term used in the diamond industry to refer to shards of gem-grade/quality diamonds. In the manufacturing and heavy industries, "bort" is used to describe dark, imperfectly formed/crystallized diamonds of varying levels of opacity. The lowest grade, "crushing bort", is crushed by...
, are destined for industrial use. In addition to mined diamonds, synthetic diamond
Synthetic diamond
Synthetic diamond is diamond produced in a technological process; as opposed to natural diamond, which is created in geological processes. Synthetic diamond is also widely known as HPHT diamond or CVD diamond, denoting the production method, High-Pressure High-Temperature synthesis and Chemical...
s found industrial applications almost immediately after their invention in the 1950s; another 400 million carats (80 tonnes) of synthetic diamonds are produced annually for industrial use—nearly four times the mass of natural diamonds mined over the same period.
The dominant industrial use of diamond is in cutting, drilling (drill bits), grinding (diamond edged cutters), and polishing. Most uses of diamonds in these technologies do not require large diamonds; in fact, most diamonds that are gem-quality can find an industrial use. Diamonds are embedded in drill tips or saw blades, or ground into a powder for use in grinding and polishing applications. Specialized applications include use in laboratories as containment for high pressure experiments (see diamond anvil), high-performance bearings
Bearing (mechanical)
A bearing is a device to allow constrained relative motion between two or more parts, typically rotation or linear movement. Bearings may be classified broadly according to the motions they allow and according to their principle of operation as well as by the directions of applied loads they can...
, and limited use in specialized window
Window
A window is a transparent or translucent opening in a wall or door that allows the passage of light and, if not closed or sealed, air and sound. Windows are usually glazed or covered in some other transparent or translucent material like float glass. Windows are held in place by frames, which...
s.
With the continuing advances being made in the production of synthetic diamond, future applications are beginning to become feasible. Garnering much excitement is the possible use of diamond as a semiconductor
Semiconductor
A semiconductor is a material with electrical conductivity due to electron flow intermediate in magnitude between that of a conductor and an insulator. This means a conductivity roughly in the range of 103 to 10−8 siemens per centimeter...
suitable to build microchip
Integrated circuit
An integrated circuit or monolithic integrated circuit is an electronic circuit manufactured by the patterned diffusion of trace elements into the surface of a thin substrate of semiconductor material...
s from, or the use of diamond as a heat sink
Heat sink
A heat sink is a term for a component or assembly that transfers heat generated within a solid material to a fluid medium, such as air or a liquid. Examples of heat sinks are the heat exchangers used in refrigeration and air conditioning systems and the radiator in a car...
in electronics
Electronics
Electronics is the branch of science, engineering and technology that deals with electrical circuits involving active electrical components such as vacuum tubes, transistors, diodes and integrated circuits, and associated passive interconnection technologies...
. Significant research efforts in Japan
Japan
Japan is an island nation in East Asia. Located in the Pacific Ocean, it lies to the east of the Sea of Japan, China, North Korea, South Korea and Russia, stretching from the Sea of Okhotsk in the north to the East China Sea and Taiwan in the south...
, Europe
Europe
Europe is, by convention, one of the world's seven continents. Comprising the westernmost peninsula of Eurasia, Europe is generally 'divided' from Asia to its east by the watershed divides of the Ural and Caucasus Mountains, the Ural River, the Caspian and Black Seas, and the waterways connecting...
, and the United States
United States
The United States of America is a federal constitutional republic comprising fifty states and a federal district...
are under way to capitalize on the potential offered by diamond's unique material properties, combined with increased quality and quantity of supply starting to become available from synthetic diamond manufacturers.
Each carbon atom in a diamond is covalently bonded to four other carbons in a tetrahedron
Tetrahedron
In geometry, a tetrahedron is a polyhedron composed of four triangular faces, three of which meet at each vertex. A regular tetrahedron is one in which the four triangles are regular, or "equilateral", and is one of the Platonic solids...
. These tetrahedrons together form a 3-dimensional network of six-membered carbon rings (similar to cyclohexane
Cyclohexane
Cyclohexane is a cycloalkane with the molecular formula C6H12. Cyclohexane is used as a nonpolar solvent for the chemical industry, and also as a raw material for the industrial production of adipic acid and caprolactam, both of which being intermediates used in the production of nylon...
), in the chair conformation, allowing for zero bond angle strain. This stable network of covalent bonds and hexagonal rings, is the reason that diamond is so incredibly strong.
Graphite
Graphite (named by Abraham Gottlob WernerAbraham Gottlob Werner
Abraham Gottlob Werner , was a German geologist who set out an early theory about the stratification of the Earth's crust and coined the word Neptunism...
in 1789, from the Greek γράφειν (graphein, "to draw/write", for its use in pencils) is one of the most common allotropes of carbon. Unlike diamond, graphite is an electrical conductor. Thus, it can be used in, for instance, electrical arc lamp electrodes. Likewise, under standard conditions, graphite is the most stable form of carbon. Therefore, it is used in thermochemistry as the standard state
Standard state
In chemistry, the standard state of a material is a reference point used to calculate its properties under different conditions. In principle, the choice of standard state is arbitrary, although the International Union of Pure and Applied Chemistry recommends a conventional set of standard states...
for defining the heat of formation of carbon compounds.
Graphite conducts electricity
Electrical conductor
In physics and electrical engineering, a conductor is a material which contains movable electric charges. In metallic conductors such as copper or aluminum, the movable charged particles are electrons...
, due to delocalization of the pi bond
Pi bond
In chemistry, pi bonds are covalent chemical bonds where two lobes of one involved atomic orbital overlap two lobes of the other involved atomic orbital...
electrons above and below the planes of the carbon atoms. These electrons are free to move, so are able to conduct electricity. However, the electricity is only conducted along the plane of the layers. In diamond, all four outer electrons of each carbon atom are 'localised' between the atoms in covalent bonding. The movement of electrons is restricted and diamond does not conduct an electric current. In graphite, each carbon atom uses only 3 of its 4 outer energy level electrons in covalently bonding to three other carbon atoms in a plane. Each carbon atom contributes one electron to a delocalised system of electrons that is also a part of the chemical bonding. The delocalised electrons are free to move throughout the plane. For this reason, graphite conducts electricity along the planes of carbon atoms, but does not conduct in a direction at right angles to the plane.
Graphite powder is used as a dry lubricant
Lubricant
A lubricant is a substance introduced to reduce friction between moving surfaces. It may also have the function of transporting foreign particles and of distributing heat...
. Although it might be thought that this industrially important property is due entirely to the loose interlamellar coupling
Cleavage (crystal)
Cleavage, in mineralogy, is the tendency of crystalline materials to split along definite crystallographic structural planes. These planes of relative weakness are a result of the regular locations of atoms and ions in the crystal, which create smooth repeating surfaces that are visible both in the...
between sheets in the structure, in fact in a vacuum
Vacuum
In everyday usage, vacuum is a volume of space that is essentially empty of matter, such that its gaseous pressure is much less than atmospheric pressure. The word comes from the Latin term for "empty". A perfect vacuum would be one with no particles in it at all, which is impossible to achieve in...
environment (such as in technologies for use in space
Outer space
Outer space is the void that exists between celestial bodies, including the Earth. It is not completely empty, but consists of a hard vacuum containing a low density of particles: predominantly a plasma of hydrogen and helium, as well as electromagnetic radiation, magnetic fields, and neutrinos....
), graphite was found to be a very poor lubricant. This fact led to the discovery that graphite's lubricity is due to adsorbed air and water between the layers, unlike other layered dry lubricants such as molybdenum disulfide
Molybdenum disulfide
Molybdenum disulfide is the inorganic compound with the formula MoS2. This black crystalline sulfide of molybdenum occurs as the mineral molybdenite. It is the principal ore from which molybdenum metal is extracted. The natural amorphous form is known as the rarer mineral jordisite. MoS2 is less...
. Recent studies suggest that an effect called superlubricity
Superlubricity
Superlubricity is a regime of motion in which friction vanishes or very nearly vanishes.Superlubricity may occur when two crystalline surfaces slide over each other in dry incommensurate contact...
can also account for this effect.
When a large number of crystallographic defects bind these planes together, graphite loses its lubrication properties and becomes what is known as pyrolytic carbon
Pyrolytic carbon
Pyrolytic carbon is a material similar to graphite, but with some covalent bonding between its graphene sheets as a result of imperfections in its production....
, a useful material in blood-contacting implants such as prosthetic heart valve
Heart valve
A heart valve normally allows blood flow in only one direction through the heart. The four valves commonly represented in a mammalian heart determine the pathway of blood flow through the heart...
s.
Natural and crystalline graphites are not often used in pure form as structural materials due to their shear-planes, brittleness and inconsistent mechanical properties.
In its pure glassy (isotropic) synthetic forms, pyrolytic graphite and carbon fiber
Carbon fiber
Carbon fiber, alternatively graphite fiber, carbon graphite or CF, is a material consisting of fibers about 5–10 μm in diameter and composed mostly of carbon atoms. The carbon atoms are bonded together in crystals that are more or less aligned parallel to the long axis of the fiber...
graphite are extremely strong, heat-resistant (to 3000 °C) materials, used in reentry shields for missile nosecones, solid rocket
Solid rocket
A solid rocket or a solid-fuel rocket is a rocket engine that uses solid propellants . The earliest rockets were solid-fuel rockets powered by gunpowder; they were used by the Chinese in warfare as early as the 13th century and later by the Mongols, Arabs, and Indians.All rockets used some form of...
engines, high temperature reactors
Pebble bed reactor
The pebble bed reactor is a graphite-moderated, gas-cooled, nuclear reactor. It is a type of very high temperature reactor , one of the six classes of nuclear reactors in the Generation IV initiative...
, brake
Brake
A brake is a mechanical device which inhibits motion. Its opposite component is a clutch. The rest of this article is dedicated to various types of vehicular brakes....
shoes and electric motor
Electric motor
An electric motor converts electrical energy into mechanical energy.Most electric motors operate through the interaction of magnetic fields and current-carrying conductors to generate force...
brushes.
Intumescent or expandable graphites are used in fire seals, fitted around the perimeter of a fire door. During a fire the graphite intumesces (expands and chars) to resist fire penetration and prevent the spread of fumes. A typical start expansion temperature (SET) is between 150 and 300 °C.
Density: its specific gravity is 2.3, which makes it lighter than diamond.
Effect of heat: it is the most stable allotrope of carbon. At high temperatures and pressures (roughly 2000 °C and 5 GPa), it can be transformed into diamond. At about 700 °C it burns in oxygen forming carbon dioxide.
Chemical activity: it is slightly more reactive than diamond. This is because the reactants are able to penetrate between the hexagonal layers of carbon atoms in graphite. It is unaffected by ordinary solvents, dilute acids, or fused alkalis. However, chromic acid
Chromic acid
The term chromic acid is usually used for a mixture made by adding concentrated sulfuric acid to a dichromate, which may contain a variety of compounds, including solid chromium trioxide. This kind of chromic acid may be used as a cleaning mixture for glass. Chromic acid may also refer to the...
oxidises it to carbon dioxide.
Graphene
A single layer of graphite is called grapheneGraphene
Graphene is an allotrope of carbon, whose structure is one-atom-thick planar sheets of sp2-bonded carbon atoms that are densely packed in a honeycomb crystal lattice. The term graphene was coined as a combination of graphite and the suffix -ene by Hanns-Peter Boehm, who described single-layer...
and has extraordinary electrical, thermal, and physical properties. It can be produced by epitaxy on an insulating or conducting substrate or by mechanical exfoliation (repeated peeling) from graphite. Its applications may include replacing silicon in high-performance electronic devices.
Amorphous carbon
Amorphous carbon is the name used for carbonCarbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
that does not have any crystal
Crystal
A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
line structure. As with all glassy
Amorphous solid
In condensed matter physics, an amorphous or non-crystalline solid is a solid that lacks the long-range order characteristic of a crystal....
materials, some short-range order can be observed, but there is no long-range pattern of atomic positions. While entirely amorphous carbon can be produced, most amorphous carbon actually contains microscopic crystals of graphite
Graphite
The mineral graphite is one of the allotropes of carbon. It was named by Abraham Gottlob Werner in 1789 from the Ancient Greek γράφω , "to draw/write", for its use in pencils, where it is commonly called lead . Unlike diamond , graphite is an electrical conductor, a semimetal...
-like, or even diamond
Diamond
In mineralogy, diamond is an allotrope of carbon, where the carbon atoms are arranged in a variation of the face-centered cubic crystal structure called a diamond lattice. Diamond is less stable than graphite, but the conversion rate from diamond to graphite is negligible at ambient conditions...
-like carbon.
Coal
Coal
Coal is a combustible black or brownish-black sedimentary rock usually occurring in rock strata in layers or veins called coal beds or coal seams. The harder forms, such as anthracite coal, can be regarded as metamorphic rock because of later exposure to elevated temperature and pressure...
and soot
Soot
Soot is a general term that refers to impure carbon particles resulting from the incomplete combustion of a hydrocarbon. It is more properly restricted to the product of the gas-phase combustion process but is commonly extended to include the residual pyrolyzed fuel particles such as cenospheres,...
or carbon black
Carbon black
Carbon black is a material produced by the incomplete combustion of heavy petroleum products such as FCC tar, coal tar, ethylene cracking tar, and a small amount from vegetable oil. Carbon black is a form of amorphous carbon that has a high surface-area-to-volume ratio, although its...
are informally called amorphous carbon. However, they are products of pyrolysis
Pyrolysis
Pyrolysis is a thermochemical decomposition of organic material at elevated temperatures without the participation of oxygen. It involves the simultaneous change of chemical composition and physical phase, and is irreversible...
(the process of decomposing a substance by the action of heat), which does not produce true amorphous carbon under normal conditions. The coal industry divides coal up into various grades depending on the amount of carbon present in the sample compared to the amount of impurities. The highest grade, anthracite
Anthracite coal
Anthracite is a hard, compact variety of mineral coal that has a high luster...
, is about 90% carbon and 10% other elements. Bituminous coal
Bituminous coal
Bituminous coal or black coal is a relatively soft coal containing a tarlike substance called bitumen. It is of higher quality than lignite coal but of poorer quality than Anthracite...
is about 75–90% carbon, and lignite
Lignite
Lignite, often referred to as brown coal, or Rosebud coal by Northern Pacific Railroad,is a soft brown fuel with characteristics that put it somewhere between coal and peat...
is the name for coal that is around 55% carbon.
Buckminsterfullerenes
The buckminsterfullerenes, or usually just fullerenes or buckyballs for short, were discovered in 1985 by a team of scientists from Rice University and the University of Sussex, three of whom were awarded the 1996 Nobel Prize in Chemistry. They are named for the resemblance of their alliotropic structure to the geodesic structures devised by the scientist and architect Richard Buckminster "Bucky" FullerBuckminster Fuller
Richard Buckminster “Bucky” Fuller was an American systems theorist, author, designer, inventor, futurist and second president of Mensa International, the high IQ society....
. Fullerenes are molecules of varying sizes composed entirely of carbon, which take the form of a hollow sphere, ellipsoid, or tube.
As of the early twenty-first century, the chemical and physical properties of fullerenes are still under heavy study, in both pure and applied research labs. In April 2003, fullerenes were under study for potential medicinal use — binding specific antibiotics to the structure to target resistant bacteria and even target certain cancer cells such as melanoma.
Carbon nanotubes
Carbon nanotubes, also called buckytubes, are cylindrical carbonCarbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
molecules with novel properties that make them potentially useful in a wide variety of applications (e.g., nano-electronics, optics
Optics
Optics is the branch of physics which involves the behavior and properties of light, including its interactions with matter and the construction of instruments that use or detect it. Optics usually describes the behavior of visible, ultraviolet, and infrared light...
, materials applications, etc.). They exhibit extraordinary strength, unique electrical
Electricity
Electricity is a general term encompassing a variety of phenomena resulting from the presence and flow of electric charge. These include many easily recognizable phenomena, such as lightning, static electricity, and the flow of electrical current in an electrical wire...
properties, and are efficient conductors of heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
. Inorganic nanotubes have also been synthesized.
A nanotube is a member of the fullerene
Fullerene
A fullerene is any molecule composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Spherical fullerenes are also called buckyballs, and they resemble the balls used in association football. Cylindrical ones are called carbon nanotubes or buckytubes...
structural family, which also includes buckyballs. Whereas buckyballs are spherical in shape, a nanotube is cylindrical
Cylinder (geometry)
A cylinder is one of the most basic curvilinear geometric shapes, the surface formed by the points at a fixed distance from a given line segment, the axis of the cylinder. The solid enclosed by this surface and by two planes perpendicular to the axis is also called a cylinder...
, with at least one end typically capped with a hemisphere of the buckyball structure. Their name is derived from their size, since the diameter of a nanotube is on the order of a few nanometers (approximately 50,000 times smaller than the width of a human hair), while they can be up to several centimeters in length. There are two main types of nanotubes: single-walled nanotubes (SWNTs) and multi-walled nanotubes
Mwnt
Mwnt is a very small community and ancient parish in south Ceredigion, Wales, on the Irish Sea coast about 4.5 miles from Cardigan. It gets its name from the prominent steep conical hill, a landmark from much of Cardigan Bay, that rises above the beach....
(MWNTs).
Carbon nanobuds
Carbon nanobuds are a newly discovered allotrope of carbonCarbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
in which fullerene
Fullerene
A fullerene is any molecule composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Spherical fullerenes are also called buckyballs, and they resemble the balls used in association football. Cylindrical ones are called carbon nanotubes or buckytubes...
like "buds" are covalently attached to the outer sidewalls of the carbon nanotubes. This hybrid material has useful properties of both fullerenes and carbon nanotubes. For instance, they have been found to be exceptionally good field emitters
Field emission
Field emission is emission of electrons induced by an electrostatic field. The most common context is FE from a solid surface into vacuum. However, FE can take place from solid or liquid surfaces, into vacuum, air, a fluid, or any non-conducting or weakly-conducting dielectric...
.
Glassy carbon
Glassy carbon or vitreous carbon is a class of non-graphitizing carbonCarbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
widely used as an electrode material in electrochemistry
Electrochemistry
Electrochemistry is a branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor and an ionic conductor , and which involve electron transfer between the electrode and the electrolyte or species in solution.If a chemical reaction is...
, as well as for high temperature crucibles and as a component of some prosthetic devices.
It was first produced by Bernard Redfern in the mid 1950s at the laboratories of The Carborundum Company, Manchester, UK. He had set out to develop a polymer matrix to mirror a diamond structure and discovered a resole (phenolic) resin that would, with special preparation, set without a catalyst. Using this resin the first glassy carbon was produced.
The preparation of glassy carbon involves subjecting the organic precursors to a series of heat treatments at temperatures up to 3000 °C. Unlike many non-graphitizing carbons, they are impermeable to gases and are chemically extremely inert, especially those prepared at very high temperatures. It has been demonstrated that the rates of oxidation of certain glassy carbons in oxygen, carbon dioxide or water vapour are lower than those of any other carbon. They are also highly resistant to attack by acids. Thus, while normal graphite
Graphite
The mineral graphite is one of the allotropes of carbon. It was named by Abraham Gottlob Werner in 1789 from the Ancient Greek γράφω , "to draw/write", for its use in pencils, where it is commonly called lead . Unlike diamond , graphite is an electrical conductor, a semimetal...
is reduced to a powder by a mixture of concentrated sulfuric and nitric acids at room temperature, glassy carbon is unaffected by such treatment, even after several months.
Atomic and diatomic carbon
Under certain conditions, carbon can be found in its atomic form. It is formed by passing large electric currents through carbon under very low pressures. It is extremely unstable, but it is an intermittent product used in the creation of carbeneCarbene
In chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
s.
Diatomic carbon can also be found under certain conditions. It is often detected via spectroscopy
Spectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
in extraterrestrial bodies, including comet
Comet
A comet is an icy small Solar System body that, when close enough to the Sun, displays a visible coma and sometimes also a tail. These phenomena are both due to the effects of solar radiation and the solar wind upon the nucleus of the comet...
s and certain star
Star
A star is a massive, luminous sphere of plasma held together by gravity. At the end of its lifetime, a star can also contain a proportion of degenerate matter. The nearest star to Earth is the Sun, which is the source of most of the energy on Earth...
s.
Carbon nanofoam
Carbon nanofoam is the fifth known allotrope of carbon discovered in 1997 by Andrei V. Rode and co-workers at the Australian National UniversityAustralian National University
The Australian National University is a teaching and research university located in the Australian capital, Canberra.As of 2009, the ANU employs 3,945 administrative staff who teach approximately 10,000 undergraduates, and 7,500 postgraduate students...
in Canberra
Canberra
Canberra is the capital city of Australia. With a population of over 345,000, it is Australia's largest inland city and the eighth-largest city overall. The city is located at the northern end of the Australian Capital Territory , south-west of Sydney, and north-east of Melbourne...
. It consists of a low-density cluster-assembly of carbon atoms strung together in a loose three-dimensional web.
Each cluster is about 6 nanometers wide and consists of about 4000 carbon atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s linked in graphite
Graphite
The mineral graphite is one of the allotropes of carbon. It was named by Abraham Gottlob Werner in 1789 from the Ancient Greek γράφω , "to draw/write", for its use in pencils, where it is commonly called lead . Unlike diamond , graphite is an electrical conductor, a semimetal...
-like sheets that are given negative curvature by the inclusion of heptagons among the regular hexagonal pattern. This is the opposite of what happens in the case of buckminsterfullerene
Buckminsterfullerene
Buckminsterfullerene is a spherical fullerene molecule with the formula . It was first intentionally prepared in 1985 by Harold Kroto, James Heath, Sean O'Brien, Robert Curl and Richard Smalley at Rice University...
s, in which carbon sheets are given positive curvature by the inclusion of pentagon
Pentagon
In geometry, a pentagon is any five-sided polygon. A pentagon may be simple or self-intersecting. The sum of the internal angles in a simple pentagon is 540°. A pentagram is an example of a self-intersecting pentagon.- Regular pentagons :In a regular pentagon, all sides are equal in length and...
s.
The large-scale structure of carbon nanofoam is similar to that of an aerogel
Aerogel
Aerogel is a synthetic porous material derived from a gel, in which the liquid component of the gel has been replaced with a gas. The result is a solid with extremely low density and thermal conductivity...
, but with 1% of the density of previously produced carbon aerogels – only a few times the density of air at sea level
Sea level
Mean sea level is a measure of the average height of the ocean's surface ; used as a standard in reckoning land elevation...
. Unlike carbon aerogels, carbon nanofoam is a poor electrical conductor.
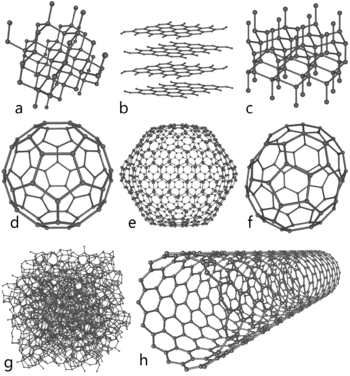
Lonsdaleite (hexagonal diamond)
Lonsdaleite is a hexagonalHexagonal crystal system
In crystallography, the hexagonal crystal system is one of the 7 crystal systems, the hexagonal lattice system is one of the 7 lattice systems, and the hexagonal crystal family is one of the 6 crystal families...
allotrope of the carbon allotrope diamond
Diamond
In mineralogy, diamond is an allotrope of carbon, where the carbon atoms are arranged in a variation of the face-centered cubic crystal structure called a diamond lattice. Diamond is less stable than graphite, but the conversion rate from diamond to graphite is negligible at ambient conditions...
, believed to form from graphite
Graphite
The mineral graphite is one of the allotropes of carbon. It was named by Abraham Gottlob Werner in 1789 from the Ancient Greek γράφω , "to draw/write", for its use in pencils, where it is commonly called lead . Unlike diamond , graphite is an electrical conductor, a semimetal...
present in meteor
METEOR
METEOR is a metric for the evaluation of machine translation output. The metric is based on the harmonic mean of unigram precision and recall, with recall weighted higher than precision...
ites upon their impact to Earth
Earth
Earth is the third planet from the Sun, and the densest and fifth-largest of the eight planets in the Solar System. It is also the largest of the Solar System's four terrestrial planets...
. The great heat and stress of the impact transforms the graphite into diamond, but retains graphite's hexagonal crystal
Crystal
A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
lattice. Hexagonal diamond has also been synthesized in the laboratory, by compressing and heating graphite either in a static press or using explosives. It can also be produced by the thermal decomposition of a polymer, poly(hydridocarbyne)
Poly(hydridocarbyne)
PolyFormula[HC]nMolecular mass200,000 to 100 million DaltonsMelting pointdecomposes @ 100°CBoiling point N/A Density??.?? g/cm³CAS number???-??-?SMILES???????...
, at atmospheric pressure, under inert gas atmosphere (e.g. argon, nitrogen), starting at temperature 110 °C (230 °F).
Linear acetylenic carbon (LAC)
A one-dimensional carbon polymer with the structure -(C:::C)n-.Other possible forms
- ChaoiteChaoiteChaoite or white carbon is a mineral described as an allotrope of carbon whose existence is disputed. It was discovered in shock-fused graphite gneiss from the Ries crater in Bavaria. It has been described as slightly harder than graphite, with a reflection colour of grey to white...
is a mineral believed to have been formed in meteorite impacts. It has been described as slightly harder than graphite with a reflection colour of grey to white. However, the existence of carbyne phases is disputed – see the entry on chaoiteChaoiteChaoite or white carbon is a mineral described as an allotrope of carbon whose existence is disputed. It was discovered in shock-fused graphite gneiss from the Ries crater in Bavaria. It has been described as slightly harder than graphite, with a reflection colour of grey to white...
for details.
- Metallic carbon: Theoretical studies have shown that there are regions in the phase diagramPhase diagramA phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions at which thermodynamically distinct phases can occur at equilibrium...
, at extremely high pressures, where carbon has metallic character.
- bcc-carbon: At ultrahigh pressures of above 1000 GPa, diamond is predicted to transform into the so-called C8 structure, a body-centered cubic structure with 8 atoms in the unit cell. This cubic carbon phase might have importance in astrophysics. Its structure is known in one of the metastable phases of silicon and is similar to cubaneCubaneCubane is a synthetic hydrocarbon molecule that consists of eight carbon atoms arranged at the corners of a cube, with one hydrogen atom attached to each carbon atom. A solid crystalline substance, cubane is one of the Platonic hydrocarbons. It was first synthesized in 1964 by Philip Eaton, a...
. Superdense and superhard material resembling this phase has been synthesized and published in 2008.
- bct-carbon: Body-centered tetragonal carbon is a very hard material produced from graphite under high pressure at room temperature. It was first thought to have been created in 2003 and additional research in 2010 appears to confirm this.
- There is an evidence that white dwarfWhite dwarfA white dwarf, also called a degenerate dwarf, is a small star composed mostly of electron-degenerate matter. They are very dense; a white dwarf's mass is comparable to that of the Sun and its volume is comparable to that of the Earth. Its faint luminosity comes from the emission of stored...
stars have a core of crystallized carbon and oxygen nuclei. The largest of these found in the universe so far, BPM 37093BPM 37093BPM 37093 is a variable white dwarf star of the DAV, or ZZ Ceti, type, with a hydrogen atmosphere and an unusually high mass of approximately 1.1 times the Sun's. It is about 50 light-years from Earth, in the constellation Centaurus, and vibrates; these pulsations cause its luminosity to vary...
, is located 50 ly away in the constellation CentaurusCentaurusCentaurus is a bright constellation in the southern sky. One of the largest constellations, Centaurus was included among the 48 constellations listed by the 2nd century astronomer Ptolemy, and it remains one of the 88 modern constellations.-Stars:...
. A news release from the Harvard-Smithsonian Center for AstrophysicsHarvard-Smithsonian Center for AstrophysicsThe Harvard–Smithsonian Center for Astrophysics is one of the largest and most diverse astrophysical institutions in the world, where scientists carry out a broad program of research in astronomy, astrophysics, earth and space sciences, and science education...
described the 2500 miles (4,023.4 km)-wide stellar core as a diamond, and it was named as Lucy, after the Beatles' song "Lucy in the Sky With Diamonds"; however, it is more likely an exotic form of carbon.
- Prismane C8 is a theoretically-predicted metastable carbon allotrope comprising an atomic clusterCluster chemistryIn chemistry, a cluster is an ensemble of bound atoms intermediate in size between a molecule and a bulk solid. Clusters exist of diverse stoichiometries and nuclearities. For example, carbon and boron atoms form fullerene and borane clusters, respectively. Transition metals and main group...
of eight carbon atoms, with the shape of an elongated triangular bipyramid—a six-atom triangular prismPrism (geometry)In geometry, a prism is a polyhedron with an n-sided polygonal base, a translated copy , and n other faces joining corresponding sides of the two bases. All cross-sections parallel to the base faces are the same. Prisms are named for their base, so a prism with a pentagonal base is called a...
with two more atoms above and below its bases.
Variability of carbon
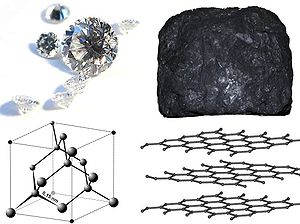
Between diamond and graphite:
- Diamond crystallizes in the cubic system but graphite crystallizes in the hexagonal system.
- Diamond is clear and transparent, but graphite is black and opaque
- Diamond is hardest mineral known (10 on the Mohs scaleMohs scale of mineral hardnessThe Mohs scale of mineral hardness characterizes the scratch resistance of various minerals through the ability of a harder material to scratch a softer material. It was created in 1812 by the German geologist and mineralogist Friedrich Mohs and is one of several definitions of hardness in...
), but graphite is one of the softest (1–2 on Mohs scaleMohs scale of mineral hardnessThe Mohs scale of mineral hardness characterizes the scratch resistance of various minerals through the ability of a harder material to scratch a softer material. It was created in 1812 by the German geologist and mineralogist Friedrich Mohs and is one of several definitions of hardness in...
). - Diamond is the ultimate abrasive, but graphite is soft and is a very good lubricant.
- Diamond is an excellent electrical insulator, but graphite is a conductor of electricity.
- Diamond is an excellent thermal conductor, but some forms of graphite are used for thermal insulation (for example heat shields and firebreaks)
- At standard temperature and pressure, graphite is the more thermodynamically stable form; the conversion, however, has a very high activation energyActivation energyIn chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
and is thus extremely slow
Despite the hardness of diamonds, the chemical bonds that hold the carbon atoms in diamonds together are actually weaker than those that hold together graphite. The difference is that in diamond, the bonds form an inflexible three-dimensional lattice. In graphite, the atoms are tightly bonded into sheets, but the sheets can slide easily making graphite soft.
External links
- http://www.dendritics.com/scales/c-allotropes.asp
- http://cst-www.nrl.navy.mil/lattice/struk/carbon.html
- diamond 3D animation

