
Synthetic diamond
Encyclopedia

Diamond
In mineralogy, diamond is an allotrope of carbon, where the carbon atoms are arranged in a variation of the face-centered cubic crystal structure called a diamond lattice. Diamond is less stable than graphite, but the conversion rate from diamond to graphite is negligible at ambient conditions...
produced in a technological
Technology
Technology is the making, usage, and knowledge of tools, machines, techniques, crafts, systems or methods of organization in order to solve a problem or perform a specific function. It can also refer to the collection of such tools, machinery, and procedures. The word technology comes ;...
process; as opposed to natural diamond, which is created in geological
Geology
Geology is the science comprising the study of solid Earth, the rocks of which it is composed, and the processes by which it evolves. Geology gives insight into the history of the Earth, as it provides the primary evidence for plate tectonics, the evolutionary history of life, and past climates...
processes. Synthetic diamond is also widely known as HPHT diamond or CVD diamond
Chemical vapor deposition
Chemical vapor deposition is a chemical process used to produce high-purity, high-performance solid materials. The process is often used in the semiconductor industry to produce thin films. In a typical CVD process, the wafer is exposed to one or more volatile precursors, which react and/or...
, denoting the production method, High-Pressure High-Temperature synthesis and Chemical Vapor Deposition, respectively.
Numerous claims of diamond synthesis were documented between 1879 and 1928; most of those attempts were carefully analyzed but none were confirmed. In the 1940s, systematic research began in the United States, Sweden and the Soviet Union
Soviet Union
The Soviet Union , officially the Union of Soviet Socialist Republics , was a constitutionally socialist state that existed in Eurasia between 1922 and 1991....
to grow diamonds using CVD and HPHT processes. The first reproducible synthesis was reported around 1953. Those two processes still dominate the production of synthetic diamond. A third method, known as detonation
Detonation nanodiamond
Detonation nanodiamond , often also called ultradispersed diamond , is diamond that originates from a detonation. When an oxygen-deficient explosive mixture of TNT/RDX is detonated in a closed chamber, diamond particles with a diameter of ca...
synthesis, entered the diamond market in the late 1990s. In this process, nanometer-sized diamond grains are created in a detonation of carbon-containing explosives. A fourth method, treating graphite with high-power ultrasound
Ultrasound
Ultrasound is cyclic sound pressure with a frequency greater than the upper limit of human hearing. Ultrasound is thus not separated from "normal" sound based on differences in physical properties, only the fact that humans cannot hear it. Although this limit varies from person to person, it is...
, has been demonstrated in the laboratory, but currently has no commercial application.
The properties of synthetic diamond depend on the details of the manufacturing processes, and can be inferior or superior to those of natural diamond; the hardness, thermal conductivity
Thermal conductivity
In physics, thermal conductivity, k, is the property of a material's ability to conduct heat. It appears primarily in Fourier's Law for heat conduction....
and electron mobility
Electron mobility
In solid-state physics, the electron mobility characterizes how quickly an electron can move through a metal or semiconductor, when pulled by an electric field. In semiconductors, there is an analogous quantity for holes, called hole mobility...
are superior in some synthetic diamonds (either HPHT or CVD). Consequently, synthetic diamond is widely used in abrasive
Abrasive
An abrasive is a material, often a mineral, that is used to shape or finish a workpiece through rubbing which leads to part of the workpiece being worn away...
s, in cutting and polishing tools and in heat sink
Heat sink
A heat sink is a term for a component or assembly that transfers heat generated within a solid material to a fluid medium, such as air or a liquid. Examples of heat sinks are the heat exchangers used in refrigeration and air conditioning systems and the radiator in a car...
s. Electronic applications of synthetic diamond are being developed, including high-power switch
Switch
In electronics, a switch is an electrical component that can break an electrical circuit, interrupting the current or diverting it from one conductor to another....
es at power station
Power station
A power station is an industrial facility for the generation of electric energy....
s, high-frequency field-effect transistor
Field-effect transistor
The field-effect transistor is a transistor that relies on an electric field to control the shape and hence the conductivity of a channel of one type of charge carrier in a semiconductor material. FETs are sometimes called unipolar transistors to contrast their single-carrier-type operation with...
s and light-emitting diode
Light-emitting diode
A light-emitting diode is a semiconductor light source. LEDs are used as indicator lamps in many devices and are increasingly used for other lighting...
s. Synthetic diamond detectors of ultraviolet
Ultraviolet
Ultraviolet light is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than X-rays, in the range 10 nm to 400 nm, and energies from 3 eV to 124 eV...
(UV) light or high-energy particles are used at high-energy research facilities and are available commercially. Because of its unique combination of thermal and chemical stability, low thermal expansion and high optical transparency in a wide spectral range, synthetic diamond is becoming the most popular material for optical windows in high-power CO2 lasers
Carbon dioxide laser
The carbon dioxide laser was one of the earliest gas lasers to be developed , and is still one of the most useful. Carbon dioxide lasers are the highest-power continuous wave lasers that are currently available...
and gyrotron
Gyrotron
Gyrotrons are high powered vacuum tubes which emit millimeter-wave beams by bunching electrons with cyclotron motion in a strong magnetic field. Output frequencies range from about 20 to 250 GHz, covering wavelengths from microwave to the edge of the terahertz gap. Typical output powers range from...
s.
Both CVD and HPHT diamonds can be cut into gems and various colors can be produced: clear white, yellow, brown, blue, green and orange. The appearance of synthetic gems on the market created major concerns in the diamond trading business, as a result of which special spectroscopic devices and techniques have been developed to distinguish synthetic and natural diamonds.
History
After the 1797 discovery that diamond was pure carbon, many attempts were made to convert various cheap forms of carbon into diamond. The earliest successes were reported by James Ballantyne HannayJames Ballantyne Hannay
James Ballantyne Hannay was a Scottish chemist who believed he had synthesized diamond in 1880. However, modern testing showed that the surviving samples from his experiments were natural diamond, not synthetic...
in 1879 and by Ferdinand Frédéric Henri Moissan
Henri Moissan
Ferdinand Frederick Henri Moissan was a French chemist who won the 1906 Nobel Prize in Chemistry for his work in isolating fluorine from its compounds.-Biography:...
in 1893. Their method involved heating charcoal
Charcoal
Charcoal is the dark grey residue consisting of carbon, and any remaining ash, obtained by removing water and other volatile constituents from animal and vegetation substances. Charcoal is usually produced by slow pyrolysis, the heating of wood or other substances in the absence of oxygen...
at up to 3500 °C with iron inside a carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
crucible in a furnace. Whereas Hannay used a flame-heated tube, Moissan applied his newly developed electric arc furnace
Electric arc furnace
An electric arc furnace is a furnace that heats charged material by means of an electric arc.Arc furnaces range in size from small units of approximately one ton capacity up to about 400 ton units used for secondary steelmaking...
, in which an electric arc was struck between carbon rods inside blocks of lime
Lime (mineral)
Lime is a general term for calcium-containing inorganic materials, in which carbonates, oxides and hydroxides predominate. Strictly speaking, lime is calcium oxide or calcium hydroxide. It is also the name for a single mineral of the CaO composition, occurring very rarely...
. The molten iron was then rapidly cooled by immersion in water. The contraction generated by the cooling supposedly produced the high pressure required to transform graphite into diamond. Moissan published his work in a series of articles in the 1890s.
Many other scientists tried to replicate his experiments. Sir William Crookes
William Crookes
Sir William Crookes, OM, FRS was a British chemist and physicist who attended the Royal College of Chemistry, London, and worked on spectroscopy...
claimed success in 1909 . Otto Ruff claimed in 1917 to have produced diamonds up to 7 mm in diameter, but later retracted his statement. In 1926, Dr. Willard Hershey of McPherson College
McPherson College
McPherson College was chartered in 1887 by the leaders of the Church of the Brethren. The college provides a career-oriented liberal arts education...
replicated Moissan's and Ruff's experiments, producing a synthetic diamond; that specimen is on display at the McPherson Museum
McPherson Museum
The McPherson Museum of McPherson, Kansas, is a local history museum that preserves the historical and cultural heritage of the McPherson community.-The Museum:...
in Kansas. Despite the claims of Moissan, Ruff, and Hershey, other experimenters were unable to reproduce their synthesis.
The most definitive replication attempts were performed by Sir Charles Algernon Parsons
Charles Algernon Parsons
Sir Charles Algernon Parsons OM KCB FRS was an Anglo-Irish engineer, best known for his invention of the steam turbine. He worked as an engineer on dynamo and turbine design, and power generation, with great influence on the naval and electrical engineering fields...
. A prominent scientist and engineer known for his invention of the steam turbine
Steam turbine
A steam turbine is a mechanical device that extracts thermal energy from pressurized steam, and converts it into rotary motion. Its modern manifestation was invented by Sir Charles Parsons in 1884....
, he spent about 40 years (1882–1922) and a considerable part of his fortune trying to reproduce the experiments of Moissan and Hannay, but also adapted processes of his own. Parsons was known for his painstakingly accurate approach and methodical record keeping; all his resulting samples were preserved for further analysis by an independent party. He wrote a number of articles—some of the earliest on HPHT diamond—in which he claimed to have produced small diamonds. However in 1928 he authorized Dr. C.H. Desch to publish an article in which he stated his belief that no synthetic diamonds (including those of Moissan and others) had been produced up to that date. He suggested that most diamonds that had been produced up to that point were likely synthetic spinel
Spinel
Spinel is the magnesium aluminium member of the larger spinel group of minerals. It has the formula MgAl2O4. Balas ruby is an old name for a rose-tinted variety.-Spinel group:...
.
GE diamond project

General Electric
General Electric Company , or GE, is an American multinational conglomerate corporation incorporated in Schenectady, New York and headquartered in Fairfield, Connecticut, United States...
(GE), Norton and Carborundum companies to further develop diamond synthesis. They were able to heat carbon to about 3000 °C under a pressure of 3.5 gigapascals
Pascal (unit)
The pascal is the SI derived unit of pressure, internal pressure, stress, Young's modulus and tensile strength, named after the French mathematician, physicist, inventor, writer, and philosopher Blaise Pascal. It is a measure of force per unit area, defined as one newton per square metre...
(GPa) for a few seconds. Soon thereafter the Second World War interrupted the project. It was resumed in 1951 at the Schenectady Laboratories of GE, and a high-pressure diamond group was formed with F.P. Bundy and H.M. Strong. Tracy Hall and others joined this project shortly thereafter.
The Schenectady group improved on the anvils
Diamond anvil cell
A diamond anvil cell is a device used in scientific experiments. It allows compressing a small piece of material to extreme pressures, which can exceed 3,000,000 atmospheres ....
designed by Percy Bridgman
Percy Williams Bridgman
Percy Williams Bridgman was an American physicist who won the 1946 Nobel Prize in Physics for his work on the physics of high pressures. He also wrote extensively on the scientific method and on other aspects of the philosophy of science.- Biography :Bridgman entered Harvard University in 1900,...
, who received a Nobel Prize
Nobel Prize
The Nobel Prizes are annual international awards bestowed by Scandinavian committees in recognition of cultural and scientific advances. The will of the Swedish chemist Alfred Nobel, the inventor of dynamite, established the prizes in 1895...
for his work in 1946. Bundy and Strong made the first improvements, then more were made by Hall. The GE team used tungsten carbide
Tungsten carbide
Tungsten carbide is an inorganic chemical compound containing equal parts of tungsten and carbon atoms. Colloquially, tungsten carbide is often simply called carbide. In its most basic form, it is a fine gray powder, but it can be pressed and formed into shapes for use in industrial machinery,...
anvils within a hydraulic press to squeeze the carbonaceous sample held in a catlinite
Catlinite
Catlinite is a type of argillite , usually brownish-red in color, which occurs in a matrix of Sioux quartzite. Because it is fine-grained and easily worked, it is prized by Native Americans for use in making sacred pipes such as calumets and chanunpas...
container, the finished grit being squeezed out of the container into a gasket. The team recorded diamond synthesis on one occasion, but the experiment could not be reproduced because of uncertain synthesis conditions, and the diamond was later shown to have been a natural diamond used as a seed.
Hall achieved the first commercially successful synthesis of diamond on December 16, 1954, and this was announced on February 15, 1955. His breakthrough was using a "belt" press, which was capable of producing pressures above 10 GPa and temperatures above 2000 °C. The "belt" press (see below) used a pyrophyllite
Pyrophyllite
Pyrophyllite is a phyllosilicate mineral composed of aluminium silicate hydroxide: Al2Si4O102. It occurs in two more or less distinct varieties, namely, as crystalline folia and as compact masses; distinct crystals are not known....
container in which graphite was dissolved within molten nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
, cobalt
Cobalt
Cobalt is a chemical element with symbol Co and atomic number 27. It is found naturally only in chemically combined form. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal....
or iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
. Those metals acted as a "solvent-catalyst", which both dissolved carbon and accelerated its conversion into diamond. The largest diamond he produced was 0.15 mm across; it was too small and visually imperfect for jewelry, but usable in industrial abrasives. Hall's co-workers were able to replicate his work, and the discovery was published in the major journal Nature
Nature (journal)
Nature, first published on 4 November 1869, is ranked the world's most cited interdisciplinary scientific journal by the Science Edition of the 2010 Journal Citation Reports...
. He was the first person to grow a synthetic diamond with a reproducible, verifiable and well-documented process. He left GE in 1955, and three years later developed a new apparatus for the synthesis of diamond—a tetrahedral press with four anvils—to avoid violating a U.S. Department of Commerce secrecy order on the GE patent applications. Hall received the American Chemical Society
American Chemical Society
The American Chemical Society is a scientific society based in the United States that supports scientific inquiry in the field of chemistry. Founded in 1876 at New York University, the ACS currently has more than 161,000 members at all degree-levels and in all fields of chemistry, chemical...
Award for Creative Invention for his work in diamond synthesis.
Later developments
An independent diamond synthesis was achieved on February 16, 1953 in StockholmStockholm
Stockholm is the capital and the largest city of Sweden and constitutes the most populated urban area in Scandinavia. Stockholm is the most populous city in Sweden, with a population of 851,155 in the municipality , 1.37 million in the urban area , and around 2.1 million in the metropolitan area...
by the ASEA
ASEA
Allmänna Svenska Elektriska Aktiebolaget was a Swedish industry company. It merged with the Swiss Brown, Boveri & Cie in 1988 to form Asea Brown Boveri...
(Allmänna Svenska Elektriska Aktiebolaget), one of Sweden's major electrical manufacturing companies. Starting in 1949, ASEA employed a team of five scientists and engineers as part of a top-secret diamond-making project code-named QUINTUS. The team used a bulky split-sphere apparatus designed by Baltzar von Platen and Anders Kämpe. Pressure was maintained within the device at an estimated 8.4 GPa for an hour. A few small diamonds were produced, but not of gem quality or size. The work was not reported until the 1980s. During the 1980s, a new competitor emerged in Korea, a company named Iljin Diamond; it was followed by hundreds of Chinese enterprises. Iljin Diamond allegedly accomplished diamond synthesis in 1988 by misappropriating trade secrets from GE via a Korean former GE employee.
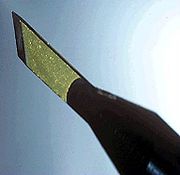
The first gem-quality stones were always yellow to brown in color because of contamination with nitrogen. Inclusions
Inclusion (mineral)
In mineralogy, an inclusion is any material that is trapped inside a mineral during its formation.In gemology, an inclusion is a characteristic enclosed within a gemstone, or reaching its surface from the interior....
were common, especially "plate-like" ones from the nickel. Removing all nitrogen from the process by adding aluminium
Aluminium
Aluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances....
or titanium
Titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. It has a low density and is a strong, lustrous, corrosion-resistant transition metal with a silver color....
produced colorless "white" stones, and removing the nitrogen and adding boron
Boron
Boron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the...
produced blue ones. Removing nitrogen also slowed the growth process and reduced the crystalline quality, so the process was normally run with nitrogen present.
Although the GE stones and natural diamonds were chemically identical, their physical properties were not the same. The colorless stones produced strong fluorescence
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. It is a form of luminescence. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation...
and phosphorescence
Phosphorescence
Phosphorescence is a specific type of photoluminescence related to fluorescence. Unlike fluorescence, a phosphorescent material does not immediately re-emit the radiation it absorbs. The slower time scales of the re-emission are associated with "forbidden" energy state transitions in quantum...
under short-wavelength ultraviolet light, but were inert under long-wave UV. Among natural diamonds, only the rarer blue gems exhibit these properties. Unlike natural diamonds, all the GE stones showed strong yellow fluorescence under X-rays. The De Beers
De Beers
De Beers is a family of companies that dominate the diamond, diamond mining, diamond trading and industrial diamond manufacturing sectors. De Beers is active in every category of industrial diamond mining: open-pit, underground, large-scale alluvial, coastal and deep sea...
Diamond Research Laboratory has grown stones of up to 25 carats (5 g) for research purposes. Stable HPHT conditions were kept for six weeks to grow high-quality diamonds of this size. For economic reasons, the growth of most synthetic diamonds is terminated when they reach a mass of 1 carats (200 mg) to 1.5 carats (300 mg).
In the 1950s, research started in the Soviet Union and the US on the growth of diamond by pyrolysis
Pyrolysis
Pyrolysis is a thermochemical decomposition of organic material at elevated temperatures without the participation of oxygen. It involves the simultaneous change of chemical composition and physical phase, and is irreversible...
of hydrocarbon gases at the relatively low temperature of 800 °C. This low-pressure process is known as chemical vapor deposition
Chemical vapor deposition
Chemical vapor deposition is a chemical process used to produce high-purity, high-performance solid materials. The process is often used in the semiconductor industry to produce thin films. In a typical CVD process, the wafer is exposed to one or more volatile precursors, which react and/or...
(CVD). William G. Eversole reportedly achieved vapor deposition of diamond over diamond substrate in 1953, but it was not reported until 1962. Diamond film deposition was independently reproduced by Angus and coworkers in 1968 and by Deryagin and Fedoseev in 1970. Whereas Eversole and Angus used large, expensive, single-crystal diamonds as substrates, Deryagin and Fedoseev succeeded in making diamond films on non-diamond materials (silicon
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
and metals), which led to massive research on inexpensive diamond coatings in the 1980s.
Manufacturing technologies
There are several methods used to produce synthetic diamond. The original method uses high pressure and high temperature (HPHT) and is still widely used because of its relatively low cost. The process involves large presses that can weigh hundreds of tons to produce a pressure of 5 GPa at 1500 °C. The second method, using chemical vapor deposition (CVD), creates a carbon plasmaPlasma (physics)
In physics and chemistry, plasma is a state of matter similar to gas in which a certain portion of the particles are ionized. Heating a gas may ionize its molecules or atoms , thus turning it into a plasma, which contains charged particles: positive ions and negative electrons or ions...
over a substrate onto which the carbon atoms deposit to form diamond. Other methods include explosive formation (forming detonation nanodiamond
Detonation nanodiamond
Detonation nanodiamond , often also called ultradispersed diamond , is diamond that originates from a detonation. When an oxygen-deficient explosive mixture of TNT/RDX is detonated in a closed chamber, diamond particles with a diameter of ca...
s) and sonication
Sonication
thumb|right|A sonicator at the [[Weizmann Institute of Science]] during sonicationSonication is the act of applying sound energy to agitate particles in a sample, for various purposes. In the laboratory, it is usually applied using an ultrasonic bath or an ultrasonic probe, colloquially known as...
of graphite solutions.
High pressure, high temperature

BARS (Diamonds)
BARS is a high-pressure high-temperature apparatus usually used for growing or processing minerals, especially diamond. The name is a transliteration of a Russian abbreviation БАРС = Беспрессовая Аппаратура высокого давления "Разрезная Сфера"...
) press.
The original GE invention by Tracy Hall uses the belt press wherein the upper and lower anvils supply the pressure load to a cylindrical inner cell. This internal pressure is confined radially by a belt of pre-stressed steel bands. The anvils also serve as electrodes providing electrical current to the compressed cell. A variation of the belt press uses hydraulic pressure, rather than steel belts, to confine the internal pressure. Belt presses are still used today, but they are built on a much larger scale than those of the original design.
The second type of press design is the cubic press. A cubic press has six anvils which provide pressure simultaneously onto all faces of a cube-shaped volume. The first multi-anvil press design was a tetrahedral press, using four anvils to converge upon a tetrahedron-shaped volume. The cubic press was created shortly thereafter to increase the volume to which pressure could be applied. A cubic press is typically smaller than a belt press and can more rapidly achieve the pressure and temperature necessary to create synthetic diamond. However, cubic presses cannot be easily scaled up to larger volumes: the pressurized volume can be increased by using larger anvils, but this also increases the amount of force needed on the anvils to achieve the same pressure. An alternative is to decrease the surface area to volume ratio of the pressurized volume, by using more anvils to converge upon a higher-order platonic solid
Platonic solid
In geometry, a Platonic solid is a convex polyhedron that is regular, in the sense of a regular polygon. Specifically, the faces of a Platonic solid are congruent regular polygons, with the same number of faces meeting at each vertex; thus, all its edges are congruent, as are its vertices and...
, such as a dodecahedron. However, such a press would be complex and difficult to manufacture.
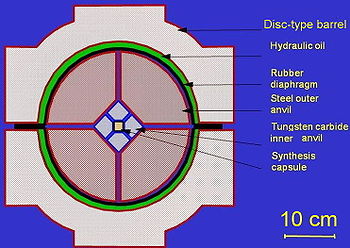
Pyrophyllite
Pyrophyllite is a phyllosilicate mineral composed of aluminium silicate hydroxide: Al2Si4O102. It occurs in two more or less distinct varieties, namely, as crystalline folia and as compact masses; distinct crystals are not known....
ceramics, which is pressed by inner anvils made from cemented carbide
Cemented carbide
Cemented carbide, also called tungsten carbide, hardmetal, or widia, is a hard material used in machining tough materials such as carbon steel or stainless steel, as well as in situations where other tools would wear away, such as high-quantity production runs. Most of the time, carbide will leave...
(e.g., tungsten carbide
Tungsten carbide
Tungsten carbide is an inorganic chemical compound containing equal parts of tungsten and carbon atoms. Colloquially, tungsten carbide is often simply called carbide. In its most basic form, it is a fine gray powder, but it can be pressed and formed into shapes for use in industrial machinery,...
or VK10 hard alloy). The outer octahedral cavity is pressed by 8 steel outer anvils. After mounting, the whole assembly is locked in a disc-type barrel with a diameter about 1 meter. The barrel is filled with oil, which pressurizes upon heating, and the oil pressure is transferred to the central cell. The synthesis capsule is heated up by a coaxial graphite heater and the temperature is measured with a thermocouple
Thermocouple
A thermocouple is a device consisting of two different conductors that produce a voltage proportional to a temperature difference between either end of the pair of conductors. Thermocouples are a widely used type of temperature sensor for measurement and control and can also be used to convert a...
.
Chemical vapor deposition
Chemical vapor depositionChemical vapor deposition
Chemical vapor deposition is a chemical process used to produce high-purity, high-performance solid materials. The process is often used in the semiconductor industry to produce thin films. In a typical CVD process, the wafer is exposed to one or more volatile precursors, which react and/or...
is a method by which diamond can be grown from a hydrocarbon gas mixture. Since the early 1980s, this method has been the subject of intensive worldwide research. Whereas the mass-production of high-quality diamond crystals make the HPHT process the more suitable choice for industrial applications, the flexibility and simplicity of CVD setups explain the popularity of CVD growth in laboratory research. The advantages of CVD diamond growth include the ability to grow diamond over large areas and on various substrates, and the fine control over the chemical impurities and thus properties of the diamond produced. Unlike HPHT, CVD process does not require high pressures, as the growth typically occurs at pressures under 27 kPa.
The CVD growth involves substrate preparation, feeding varying amounts of gases into a chamber and energizing them. The substrate preparation includes choosing an appropriate material and its crystallographic orientation; cleaning it, often with a diamond powder to abrade a non-diamond substrate; and optimizing the substrate temperature (about ) during the growth through a series of test runs. The gases always include a carbon source, typically methane
Methane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
, and hydrogen with a typical ratio of 1:99. Hydrogen is essential because it selectively etches off non-diamond carbon. The gases are ionized into chemically active radicals
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
in the growth chamber using microwave
Microwave
Microwaves, a subset of radio waves, have wavelengths ranging from as long as one meter to as short as one millimeter, or equivalently, with frequencies between 300 MHz and 300 GHz. This broad definition includes both UHF and EHF , and various sources use different boundaries...
power, a hot filament, an arc discharge
Electric arc
An electric arc is an electrical breakdown of a gas which produces an ongoing plasma discharge, resulting from a current flowing through normally nonconductive media such as air. A synonym is arc discharge. An arc discharge is characterized by a lower voltage than a glow discharge, and relies on...
, a welding torch, a laser
Laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of photons. The term "laser" originated as an acronym for Light Amplification by Stimulated Emission of Radiation...
, an electron beam, or other means.
During the growth, the chamber materials are etched off by the plasma and can incorporate into the growing diamond. In particular, CVD diamond is often contaminated by silicon originating from the silica windows of the growth chamber or from the silicon substrate. Therefore, silica windows are either avoided or moved away from the substrate. Boron-containing species in the chamber, even at very low trace levels, also make it unsuitable for the growth of pure diamond.
Detonation of explosives
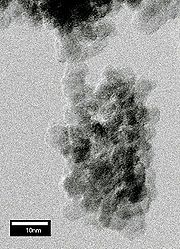
Diamond nanocrystals (5 nm in diameter) can be formed by detonating certain carbon-containing explosives in a metal chamber. These nanocrystals are called "detonation nanodiamond
Detonation nanodiamond
Detonation nanodiamond , often also called ultradispersed diamond , is diamond that originates from a detonation. When an oxygen-deficient explosive mixture of TNT/RDX is detonated in a closed chamber, diamond particles with a diameter of ca...
". During the explosion, the pressure and temperature in the chamber become high enough to convert the carbon of the explosives into diamond. Being immersed in water, the chamber cools rapidly after the explosion, suppressing conversion of newly produced diamond into more stable graphite. In a variation of this technique, a metal tube filled with graphite powder is placed in the detonation chamber. The explosion heats and compresses the graphite to an extent sufficient for its conversion into diamond. The product is always rich in graphite and other non-diamond carbon forms and requires prolonged boiling in hot nitric acid
Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
(about 1 day at 250 °C) to dissolve them. The recovered nanodiamond powder is used primarily in polishing applications. It is mainly produced in China, Russia and Belarus
Belarus
Belarus , officially the Republic of Belarus, is a landlocked country in Eastern Europe, bordered clockwise by Russia to the northeast, Ukraine to the south, Poland to the west, and Lithuania and Latvia to the northwest. Its capital is Minsk; other major cities include Brest, Grodno , Gomel ,...
and is only now beginning to reach the market in bulk quantities.
Ultrasound cavitation
MicronMicrometre
A micrometer , is by definition 1×10-6 of a meter .In plain English, it means one-millionth of a meter . Its unit symbol in the International System of Units is μm...
-sized diamond crystals can be synthesized from a suspension of graphite in organic liquid at atmospheric pressure and room temperature
Standard conditions for temperature and pressure
Standard condition for temperature and pressure are standard sets of conditions for experimental measurements established to allow comparisons to be made between different sets of data...
using ultrasonic cavitation
Cavitation
Cavitation is the formation and then immediate implosion of cavities in a liquidi.e. small liquid-free zones that are the consequence of forces acting upon the liquid...
. The diamond yield is about 10% of the initial graphite weight. The estimated cost of diamond produced by this method is comparable to that of the HPHT method; the crystalline perfection of the product is significantly worse for the ultrasonic synthesis. This technique requires relatively simple equipment and procedures, but it has only been reported by two research groups, and has no industrial use . Numerous process parameters, such as preparation of the initial graphite powder, the choice of ultrasonic power, synthesis time and the solvent, are not yet optimized, leaving a window for potential improvement of the efficiency and reduction of the cost of the ultrasonic synthesis.
Properties
Traditionally, the absence of crystal flaws is considered to be the most important quality of a diamond. Purity and high crystalline perfection make diamonds transparent and clear, whereas its hardness, optical dispersion (luster) and chemical stability (combined with marketing), make it a popular gemstone. High thermal conductivity is also important for technical applications. Whereas high optical dispersion is an intrinsic property of all diamonds, their other properties vary depending on how the diamond was created.Crystallinity
Diamond can be one single, continuous crystal or it can be made up of many smaller crystals (polycrystal). Large, clear and transparent single-crystal diamonds are typically used in gemstones. Polycrystalline diamond consists of numerous small grains, which are easily seen by the naked eye through strong light absorption and scattering; it is unsuitable for gems and is used for industrial applications such as mining and cutting tools. Polycrystalline diamond is often described by the average size (or grain sizeCrystallite
Crystallites are small, often microscopic crystals that, held together through highly defective boundaries, constitute a polycrystalline solid. Metallurgists often refer to crystallites as grains.- Details :...
) of the crystals that make it up. Grain sizes range from nanometers to hundreds of micrometer
Micrometer
A micrometer , sometimes known as a micrometer screw gauge, is a device incorporating a calibrated screw used widely for precise measurement of small distances in mechanical engineering and machining as well as most mechanical trades, along with other metrological instruments such as dial, vernier,...
s, usually referred to as "nanocrystalline" and "microcrystalline" diamond, respectively.
Hardness
Synthetic diamond is the hardest material known, where hardness is defined as resistance to scratching and is graded between 1 (softest) and 10 (hardest) using the Mohs scale of mineral hardnessMohs scale of mineral hardness
The Mohs scale of mineral hardness characterizes the scratch resistance of various minerals through the ability of a harder material to scratch a softer material. It was created in 1812 by the German geologist and mineralogist Friedrich Mohs and is one of several definitions of hardness in...
. Diamond has a hardness of 10 (hardest) on this scale. The hardness of synthetic diamond depends on its purity, crystalline perfection and orientation: hardness is higher for flawless, pure crystals oriented to the
Impurities and inclusions
Every diamond contains atoms other than carbon in concentrations detectable by analytical techniques. Those atoms can aggregate into macroscopic phases called inclusions. Impurities are generally avoided, but can be introduced intentionally as a way to control certain properties of the diamond. For instance, pure diamond is an electrical insulator, but diamond with boron added is an electrical conductor (and, in some cases, a superconductorCovalent superconductors
Covalent semiconductors are such solids as diamond, silicon, germanium, silicon carbide and silicon-germanium where atoms are linked by covalent bonds. Most of those materials, at least in their bulk form, are well studied and rarely hit the front pages of the top scientific journals in the last...
), allowing it to be used in electronic applications. Nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
impurities hinder movement of lattice dislocations (defects within the crystal structure
Crystal structure
In mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry...
) and put the lattice under compressive stress, thereby increasing hardness and toughness
Toughness
In materials science and metallurgy, toughness is the ability of a material to absorb energy and plastically deform without fracturing; Material toughness is defined as the amount of energy per volume that a material can absorb before rupturing...
.
Thermal conductivity
Unlike most electrical insulators, pure diamond is a good conductor of heat because of the strong covalent bondCovalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
ing within the crystal. The thermal conductivity of pure diamond is the highest of any known solid. Single crystals of synthetic diamond enriched in (99.9%) have the highest thermal conductivity
Thermal conductivity
In physics, thermal conductivity, k, is the property of a material's ability to conduct heat. It appears primarily in Fourier's Law for heat conduction....
of any material, 30 W/cm·K at room temperature, 7.5 times higher than copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
. Natural diamond's conductivity is reduced by 1.1 % by the naturally present, which acts as an inhomogeneity in the lattice.
Diamond's thermal conductivity is made use of by jewelers and gemologists who may employ an electronic thermal probe to separate diamonds from their imitations. These probes consist of a pair of battery-powered thermistor
Thermistor
A thermistor is a type of resistor whose resistance varies significantly with temperature, more so than in standard resistors. The word is a portmanteau of thermal and resistor...
s mounted in a fine copper tip. One thermistor functions as a heating device while the other measures the temperature of the copper tip: if the stone being tested is a diamond, it will conduct the tip's thermal energy rapidly enough to produce a measurable temperature drop. This test takes about 2–3 seconds.
Machining and cutting tools

Machine tool
A machine tool is a machine, typically powered other than by human muscle , used to make manufactured parts in various ways that include cutting or certain other kinds of deformation...
s and cutting tools. As the hardest known naturally occurring material, diamond can be used to polish, cut, or wear away any material, including other diamonds. Common industrial applications of this ability include diamond-tipped drill bit
Drill bit
Drill bits are cutting tools used to create cylindrical holes. Bits are held in a tool called a drill, which rotates them and provides torque and axial force to create the hole. Specialized bits are also available for non-cylindrical-shaped holes....
s and saws, and the use of diamond powder as an abrasive
Abrasive
An abrasive is a material, often a mineral, that is used to shape or finish a workpiece through rubbing which leads to part of the workpiece being worn away...
. These are by far the largest industrial applications of synthetic diamond. While natural diamond is also used for these purposes, synthetic HPHT diamond is more popular, mostly because of better reproducibility of its mechanical properties. Diamond is not suitable for machining ferrous
Ferrous
Ferrous , in chemistry, indicates a divalent iron compound , as opposed to ferric, which indicates a trivalent iron compound ....
alloy
Alloy
An alloy is a mixture or metallic solid solution composed of two or more elements. Complete solid solution alloys give single solid phase microstructure, while partial solutions give two or more phases that may or may not be homogeneous in distribution, depending on thermal history...
s at high speeds, as carbon is soluble in iron at the high temperatures created by high-speed machining, leading to greatly increased wear on diamond tools compared to alternatives.
The usual form of diamond in cutting tools is micrometer-sized grains dispersed in a metal matrix (usually cobalt) sintered
Sintering
Sintering is a method used to create objects from powders. It is based on atomic diffusion. Diffusion occurs in any material above absolute zero, but it occurs much faster at higher temperatures. In most sintering processes, the powdered material is held in a mold and then heated to a temperature...
onto the tool. This is typically referred to in industry as polycrystalline diamond (PCD). PCD-tipped tools can be found in mining and cutting applications. For the past fifteen years, work has been done to coat metallic tools with CVD diamond, and though the work still shows promise it has not significantly replaced traditional PCD tools.
Thermal conductor
Most materials with high thermal conductivity are also electrically conductive, such as metals. In contrast, pure synthetic diamond has high thermal conductivity, but negligible electrical conductivity. This combination is invaluable for electronics where diamond is used as a heat sinkHeat sink
A heat sink is a term for a component or assembly that transfers heat generated within a solid material to a fluid medium, such as air or a liquid. Examples of heat sinks are the heat exchangers used in refrigeration and air conditioning systems and the radiator in a car...
for high-power laser diode
Laser diode
The laser diode is a laser where the active medium is a semiconductor similar to that found in a light-emitting diode. The most common type of laser diode is formed from a p-n junction and powered by injected electric current...
s, laser arrays and high-power transistor
Transistor
A transistor is a semiconductor device used to amplify and switch electronic signals and power. It is composed of a semiconductor material with at least three terminals for connection to an external circuit. A voltage or current applied to one pair of the transistor's terminals changes the current...
s. Efficient heat dissipation prolongs the lifetime of those devices, and their high cost justifies the use of efficient, though relatively expensive, diamond heat sinks. In semiconductor technology, synthetic diamond heat spreaders prevent silicon and other semiconducting materials from overheating.
Optical material
Diamond is hard, chemically inert, and has high thermal conductivity and a low coefficient of thermal expansion. These properties make diamond superior to any other existing window material used for transmitting infrared and microwave radiation. Therefore, synthetic diamond is starting to replace zinc selenideZinc selenide
Zinc selenide , is a light yellow binary solid compound. It is an intrinsic semiconductor with a band gap of about 2.70 eV at 25 °C. ZnSe rarely occurs in nature...
as the output window of high-power CO2 lasers and gyrotron
Gyrotron
Gyrotrons are high powered vacuum tubes which emit millimeter-wave beams by bunching electrons with cyclotron motion in a strong magnetic field. Output frequencies range from about 20 to 250 GHz, covering wavelengths from microwave to the edge of the terahertz gap. Typical output powers range from...
s. Those synthetic diamond windows are shaped as disks of large diameters (about 10 cm for gyrotrons) and small thicknesses (to reduce absorption) and can only be produced with the CVD technique.
Recent advances in the HPHT and CVD synthesis techniques improved the purity and crystallographic structure perfection of single-crystalline diamond enough to replace silicon as a diffraction grating
Diffraction grating
In optics, a diffraction grating is an optical component with a periodic structure, which splits and diffracts light into several beams travelling in different directions. The directions of these beams depend on the spacing of the grating and the wavelength of the light so that the grating acts as...
and window material in high-power radiation sources, such as synchrotron
Synchrotron
A synchrotron is a particular type of cyclic particle accelerator in which the magnetic field and the electric field are carefully synchronised with the travelling particle beam. The proton synchrotron was originally conceived by Sir Marcus Oliphant...
s. Both the CVD and HPHT processes are also used to create designer optically transparent diamond anvils as a tool for measuring electric and magnetic properties of materials at ultra high pressures using a diamond anvil cell
Diamond anvil cell
A diamond anvil cell is a device used in scientific experiments. It allows compressing a small piece of material to extreme pressures, which can exceed 3,000,000 atmospheres ....
.
Electronics
Synthetic diamond has potential uses as a semiconductorSemiconductor
A semiconductor is a material with electrical conductivity due to electron flow intermediate in magnitude between that of a conductor and an insulator. This means a conductivity roughly in the range of 103 to 10−8 siemens per centimeter...
, because it can be doped with impurities like boron
Boron
Boron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the...
and phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
. Since these elements contain one more or one less valence electron
Valence electron
In chemistry, valence electrons are the electrons of an atom that can participate in the formation of chemical bonds with other atoms. Valence electrons are the "own" electrons, present in the free neutral atom, that combine with valence electrons of other atoms to form chemical bonds. In a single...
than carbon, they turn synthetic diamond into p-type
P-type semiconductor
A P-type semiconductor is obtained by carrying out a process of doping: that is, adding a certain type of atoms to the semiconductor in order to increase the number of free charge carriers ....
or n-type semiconductor
N-type semiconductor
N-type semiconductors are a type of extrinsic semiconductor where the dopant atoms are capable of providing extra conduction electrons to the host material . This creates an excess of negative electron charge carriers....
. Making a p–n junction by sequential doping of synthetic diamond with boron and phosphorus produces light-emitting diodes (LED
LEd
LEd is a TeX/LaTeX editing software working under Microsoft Windows. It is a freeware product....
s) producing UV light of 235 nm. Another useful property of synthetic diamond for electronics is high carrier mobility
Electron mobility
In solid-state physics, the electron mobility characterizes how quickly an electron can move through a metal or semiconductor, when pulled by an electric field. In semiconductors, there is an analogous quantity for holes, called hole mobility...
, which reaches 4500 cm2/(V·s) for electrons in single-crystal CVD diamond. High mobility is favorable for high-frequency field-effect transistor
Field-effect transistor
The field-effect transistor is a transistor that relies on an electric field to control the shape and hence the conductivity of a channel of one type of charge carrier in a semiconductor material. FETs are sometimes called unipolar transistors to contrast their single-carrier-type operation with...
s. The wide band gap
Band gap
In solid state physics, a band gap, also called an energy gap or bandgap, is an energy range in a solid where no electron states can exist. In graphs of the electronic band structure of solids, the band gap generally refers to the energy difference between the top of the valence band and the...
of diamond (5.5 eV) gives it excellent dielectric properties. Combined with the high mechanical stability of diamond, those properties are being used in prototype high-power switches for power stations.
Synthetic diamond transistors have been produced in the laboratory. They are functional at much higher temperatures than silicon devices, and are resistant to chemical and radiation damage. While no diamond transistors have yet been successfully integrated into commercial electronics, they are promising for use in exceptionally high power situations and hostile non-oxidizing environments.
Synthetic diamond is already used as radiation detection device
Semiconductor detector
This article is about particle detectors. For information about semiconductor detectors in radio, see Diode#Semiconductor_diodes, rectifier, detector and cat's-whisker detector....
. It is radiation hard
Radiation hardening
Radiation hardening is a method of designing and testing electronic components and systems to make them resistant to damage or malfunctions caused by ionizing radiation , such as would be encountered in outer space, high-altitude flight, around nuclear reactors, particle accelerators, or during...
and has a wide bandgap of 5.5 eV (at room temperature). Diamond is also distinguished from most other semiconductors by the lack of a stable native oxide. This makes it difficult to fabricate surface MOS devices but does create the potential for UV radiation to get to the active semiconductor without absorption in a surface layer. Because of these properties, it is employed in applications such as the BaBar
Babar
Babar means Lion. Babar may refer to:Names* Babur , 16th-century ruler of Indian subcontinent and founder of the Mughal Empire* Babar Luck, musician from England...
detector at the Stanford Linear Accelerator and BOLD (Blind to the Optical Light Detectors for VUV
Ultraviolet
Ultraviolet light is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than X-rays, in the range 10 nm to 400 nm, and energies from 3 eV to 124 eV...
solar observations). A diamond VUV detector recently was used in the European LYRA
LYRA
LYRA is the solar UV radiometer on board Proba-2, a European Space Agency technology demonstration satellite that was launched on November 2, 2009....
program.
Conductive CVD diamond is a useful electrode under many circumstances. Photochemical methods have been developed for covalently linking DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
to the surface of polycrystalline diamond films produced through CVD. Such DNA modified films can be used for detecting various biomolecule
Biomolecule
A biomolecule is any molecule that is produced by a living organism, including large polymeric molecules such as proteins, polysaccharides, lipids, and nucleic acids as well as small molecules such as primary metabolites, secondary metabolites, and natural products...
s, which would interact with DNA thereby changing electrical conductivity of the diamond film. In addition, diamonds can be used to detect redox
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
reactions that cannot ordinarily be studied and in some cases degrade redox-reactive organic contaminants in water supplies. Because diamond is mechanically and chemically stable, it can be used as an electrode under conditions that would destroy traditional materials. As an electrode, synthetic diamond can be used in waste water treatment of organic effluents and the production of strong oxidants.
Gemstones

Gemstone
A gemstone or gem is a piece of mineral, which, in cut and polished form, is used to make jewelry or other adornments...
s are grown by HPHT or CVD methods. They are available in yellow and blue, and to a lesser extent colorless (or white). The yellow color comes from nitrogen impurities in the manufacturing process while the blue color comes from boron. Other colors such as pink or green are achievable after synthesis using irradiation. Several companies also offer memorial diamonds grown using cremated remains.
Gem-quality diamonds grown in a lab can be chemically, physically and optically identical to naturally occurring ones, although they can be distinguished by spectroscopy
Spectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
in infrared
Infrared spectroscopy
Infrared spectroscopy is the spectroscopy that deals with the infrared region of the electromagnetic spectrum, that is light with a longer wavelength and lower frequency than visible light. It covers a range of techniques, mostly based on absorption spectroscopy. As with all spectroscopic...
, ultraviolet, or X-ray
X-ray spectroscopy
X-ray spectroscopy is a gathering name for several spectroscopic techniques for characterization of materials by using x-ray excitation.-Characteristic X-ray Spectroscopy:...
wavelengths. The DiamondView tester from De Beers
De Beers
De Beers is a family of companies that dominate the diamond, diamond mining, diamond trading and industrial diamond manufacturing sectors. De Beers is active in every category of industrial diamond mining: open-pit, underground, large-scale alluvial, coastal and deep sea...
uses UV fluorescence
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. It is a form of luminescence. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation...
to detect trace impurities of nitrogen, nickel or other metals in HPHT or CVD diamonds.
The mined diamond industry is evaluating marketing and distribution countermeasures to the appearance of synthetic diamonds on the gem market. The three largest distributors of mined diamonds have made public statements about selling their diamonds with full disclosure of the individual diamond history, and have implemented measures to laser
Laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of photons. The term "laser" originated as an acronym for Light Amplification by Stimulated Emission of Radiation...
-inscribe serial numbers on their gemstones.
See also
- Diamond simulantDiamond simulantThe high price of gem-grade diamonds, as well as significant ethical concerns of the diamond trade, have created a large demand for materials with similar gemological characteristics, known as diamond simulants or imitations. Simulants are distinct from synthetic diamond, which unlike simulants is...
- Isotopically pure diamondIsotopically pure diamondAn isotopically pure diamond is a type of diamond that is composed entirely of one isotope of carbon. Isotopically pure diamonds have been manufactured from either the more common carbon isotope with mass number 12 or the less common 13C isotope...
- Material properties of diamondMaterial properties of diamondDiamond is the allotrope of carbon in which the carbon atoms are arranged in the specific type of cubic lattice called diamond cubic. Diamond is an optically isotropic crystal that is transparent to opaque. Owing to its strong covalent bonding, diamond is the hardest naturally occurring material...
- MoissaniteMoissaniteMoissanite originally referred to a rare mineral discovered by Henri Moissan having a chemical formula SiC and various crystalline polymorphs. Earlier, this material had been synthesized in the laboratory and named silicon carbide .- Background :...
- Poly(hydridocarbyne)Poly(hydridocarbyne)PolyFormula[HC]nMolecular mass200,000 to 100 million DaltonsMelting pointdecomposes @ 100°CBoiling point N/A Density??.?? g/cm³CAS number???-??-?SMILES???????...

