Principle of maximum work
Encyclopedia
In the history of science
, the principle of maximum work was a postulate concerning the relationship between chemical reaction
s, heat
evolution, and the potential work produced there from. The principle was developed in approximate form in 1875 by French chemist Marcellin Berthelot
, in the field of thermochemistry
, and then later in 1876 by American mathematical physicist Willard Gibbs, in the field of thermodynamics
, in a more accurate form. Berthelot's version was essentially: "every pure chemical reaction is accompanied by evolution of heat." (and that this yields the maximum amount of work). The effects of irreversibility
, however, showed this version to be incorrect. This was rectified, in thermodynamics, by incorporating the concept of entropy
.
Thus, to summarize, in 1875 by the French chemist Marcellin Berthelot
which stated that chemical reaction
s will tend to yield the maximum amount of chemical energy in the form of work as the reaction progresses.
In 1876, however, through the works of Willard Gibbs and others to follow, the work principle was found to be a particular case of a more general statement:
The principle of maximum work was a precursor to the development of the thermodynamic concept of free energy
.
, the Gibbs free energy
or Helmholtz free energy
is essentially the energy of a chemical reaction "free" or available to do external work. Historically, the "free energy" is a more advanced and accurate replacement for the thermochemistry
term “affinity
” used by chemists of olden days to describe the “force” that caused chemical reaction
s. The term dates back to at least the time of Albertus Magnus
in 1250.
According to Nobelist and chemical engineering professor Ilya Prigogine
: “as motion was explained by the Newtonian concept of force, chemists wanted a similar concept of ‘driving force’ for chemical change? Why do chemical reactions occur, and why do they stop at certain points? Chemists called the ‘force’ that caused chemical reactions affinity, but it lacked a clear definition.
During the entire 18th century, the dominant view in regard to heat and light was that put forward by Isaac Newton
, called the “Newtonian hypothesis”, which stated that light and heat are forms of matter attracted or repelled by other forms of matter, with forces analogous to gravitation or to chemical affinity.
In the 19th century, the French chemist Marcellin Berthelot
and the Danish chemist Julius Thomsen had attempted to quantify chemical affinity
using heats of reaction. In 1875, after quantifying the heats of reaction for a large number of compounds, Berthelot proposed the “principle of maximum work” in which all chemical changes occurring without intervention of outside energy tend toward the production of bodies or of a system of bodies which liberate heat
.
 With the development of the first two laws of thermodynamics
With the development of the first two laws of thermodynamics
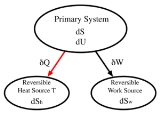
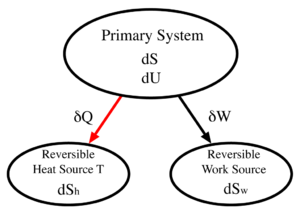
in the 1850s and 60s, heats of reaction and the work associated with these processes were given a more accurate mathematical basis. In 1876, Willard Gibbs unified all of this in his 300-page "On the Equilibrium of Heterogeneous Substances". Suppose , for example, we have a general thermodynamic system, called the "primary" system and that we mechanically connect it to a "reversible work source". A reversible work source is a system which, when it does work, or has work done to it, does not change its entropy. It is therefore not a heat engine
and does not suffer dissipation due to friction or heat exchanges. A simple example would be a frictionless spring, or a weight on a pulley in a gravitational field. Suppose further, that we thermally connect the primary system to a third system, a "reversible heat source". A reversible heat source may be thought of as a heat source in which all transformations are reversible. For such a source, the heat energy δQ added will be equal to the temperature of the source (T) times the increase in its entropy. (If it were an irreversible heat source, the entropy increase would be larger than δQ/T)
Define:
We may now make the following statements
Eliminating ,
,  , and
, and  gives the following equation:
gives the following equation:

When the primary system is reversible, the equality will hold and the amount of work delivered will be a maximum. Note that this will hold for any reversible system which has the same values of dU and dS .
History of science
The history of science is the study of the historical development of human understandings of the natural world and the domains of the social sciences....
, the principle of maximum work was a postulate concerning the relationship between chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
s, heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
evolution, and the potential work produced there from. The principle was developed in approximate form in 1875 by French chemist Marcellin Berthelot
Marcellin Berthelot
Marcelin Pierre Eugène Berthelot was a French chemist and politician noted for the Thomsen-Berthelot principle of thermochemistry. He synthesized many organic compounds from inorganic substances and disproved the theory of vitalism. He is considered as one of the greatest chemists of all time.He...
, in the field of thermochemistry
Thermochemistry
Thermochemistry is the study of the energy and heat associated with chemical reactions and/or physical transformations. A reaction may release or absorb energy, and a phase change may do the same, such as in melting and boiling. Thermochemistry focuses on these energy changes, particularly on the...
, and then later in 1876 by American mathematical physicist Willard Gibbs, in the field of thermodynamics
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
, in a more accurate form. Berthelot's version was essentially: "every pure chemical reaction is accompanied by evolution of heat." (and that this yields the maximum amount of work). The effects of irreversibility
Irreversibility
In science, a process that is not reversible is called irreversible. This concept arises most frequently in thermodynamics, as applied to processes....
, however, showed this version to be incorrect. This was rectified, in thermodynamics, by incorporating the concept of entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
.
Overview
Berthelot independently enunciated a generalization (commonly known as Berthelot's Third Principle, or Principle of Maximum Work), which may be stated in brief as follows: - Every pure chemical reaction is accompanied by evolution of heat. Whilst this principle is undoubtedly applicable to the great majority of chemical actions under ordinary conditions, it is subject to numerous exceptions, and cannot therefore be taken (as its authors originally intended) as a secure basis for theoretical reasoning on the connexion between thermal effect and chemical affinity. The existence of reactions which are reversible on slight alteration of conditions at once invalidates the principle, for if the action proceeding in one direction evolves heat, it must absorb heat when proceeding in the reverse direction. As the principle was abandoned even by its authors, it is now only of historical importance, although for many years it exerted considerable influence on thermochemical research.Thus, to summarize, in 1875 by the French chemist Marcellin Berthelot
Marcellin Berthelot
Marcelin Pierre Eugène Berthelot was a French chemist and politician noted for the Thomsen-Berthelot principle of thermochemistry. He synthesized many organic compounds from inorganic substances and disproved the theory of vitalism. He is considered as one of the greatest chemists of all time.He...
which stated that chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
s will tend to yield the maximum amount of chemical energy in the form of work as the reaction progresses.
In 1876, however, through the works of Willard Gibbs and others to follow, the work principle was found to be a particular case of a more general statement:
The principle of maximum work was a precursor to the development of the thermodynamic concept of free energy
Thermodynamic free energy
The thermodynamic free energy is the amount of work that a thermodynamic system can perform. The concept is useful in the thermodynamics of chemical or thermal processes in engineering and science. The free energy is the internal energy of a system less the amount of energy that cannot be used to...
.
Thermochemistry
In thermodynamicsThermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
, the Gibbs free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
or Helmholtz free energy
Helmholtz free energy
In thermodynamics, the Helmholtz free energy is a thermodynamic potential that measures the “useful” work obtainable from a closed thermodynamic system at a constant temperature and volume...
is essentially the energy of a chemical reaction "free" or available to do external work. Historically, the "free energy" is a more advanced and accurate replacement for the thermochemistry
Thermochemistry
Thermochemistry is the study of the energy and heat associated with chemical reactions and/or physical transformations. A reaction may release or absorb energy, and a phase change may do the same, such as in melting and boiling. Thermochemistry focuses on these energy changes, particularly on the...
term “affinity
Chemical affinity
In chemical physics and physical chemistry, chemical affinity is the electronic property by which dissimilar chemical species are capable of forming chemical compounds...
” used by chemists of olden days to describe the “force” that caused chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
s. The term dates back to at least the time of Albertus Magnus
Albertus Magnus
Albertus Magnus, O.P. , also known as Albert the Great and Albert of Cologne, is a Catholic saint. He was a German Dominican friar and a bishop, who achieved fame for his comprehensive knowledge of and advocacy for the peaceful coexistence of science and religion. Those such as James A. Weisheipl...
in 1250.
According to Nobelist and chemical engineering professor Ilya Prigogine
Ilya Prigogine
Ilya, Viscount Prigogine was a Russian-born naturalized Belgian physical chemist and Nobel Laureate noted for his work on dissipative structures, complex systems, and irreversibility.-Biography :...
: “as motion was explained by the Newtonian concept of force, chemists wanted a similar concept of ‘driving force’ for chemical change? Why do chemical reactions occur, and why do they stop at certain points? Chemists called the ‘force’ that caused chemical reactions affinity, but it lacked a clear definition.
During the entire 18th century, the dominant view in regard to heat and light was that put forward by Isaac Newton
Isaac Newton
Sir Isaac Newton PRS was an English physicist, mathematician, astronomer, natural philosopher, alchemist, and theologian, who has been "considered by many to be the greatest and most influential scientist who ever lived."...
, called the “Newtonian hypothesis”, which stated that light and heat are forms of matter attracted or repelled by other forms of matter, with forces analogous to gravitation or to chemical affinity.
In the 19th century, the French chemist Marcellin Berthelot
Marcellin Berthelot
Marcelin Pierre Eugène Berthelot was a French chemist and politician noted for the Thomsen-Berthelot principle of thermochemistry. He synthesized many organic compounds from inorganic substances and disproved the theory of vitalism. He is considered as one of the greatest chemists of all time.He...
and the Danish chemist Julius Thomsen had attempted to quantify chemical affinity
Chemical affinity
In chemical physics and physical chemistry, chemical affinity is the electronic property by which dissimilar chemical species are capable of forming chemical compounds...
using heats of reaction. In 1875, after quantifying the heats of reaction for a large number of compounds, Berthelot proposed the “principle of maximum work” in which all chemical changes occurring without intervention of outside energy tend toward the production of bodies or of a system of bodies which liberate heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
.
Thermodynamics

Laws of thermodynamics
The four laws of thermodynamics summarize its most important facts. They define fundamental physical quantities, such as temperature, energy, and entropy, in order to describe thermodynamic systems. They also describe the transfer of energy as heat and work in thermodynamic processes...
in the 1850s and 60s, heats of reaction and the work associated with these processes were given a more accurate mathematical basis. In 1876, Willard Gibbs unified all of this in his 300-page "On the Equilibrium of Heterogeneous Substances". Suppose , for example, we have a general thermodynamic system, called the "primary" system and that we mechanically connect it to a "reversible work source". A reversible work source is a system which, when it does work, or has work done to it, does not change its entropy. It is therefore not a heat engine
Heat engine
In thermodynamics, a heat engine is a system that performs the conversion of heat or thermal energy to mechanical work. It does this by bringing a working substance from a high temperature state to a lower temperature state. A heat "source" generates thermal energy that brings the working substance...
and does not suffer dissipation due to friction or heat exchanges. A simple example would be a frictionless spring, or a weight on a pulley in a gravitational field. Suppose further, that we thermally connect the primary system to a third system, a "reversible heat source". A reversible heat source may be thought of as a heat source in which all transformations are reversible. For such a source, the heat energy δQ added will be equal to the temperature of the source (T) times the increase in its entropy. (If it were an irreversible heat source, the entropy increase would be larger than δQ/T)
Define:
 |
The loss of internal energy by the primary system |
 |
The gain in entropy of the primary system |
 |
The gain in internal energy of the reversible work source |
 |
The gain in entropy of the reversible work source |
 |
The gain in internal energy of the reversible heat source |
 |
The gain in entropy of the reversible heat source |
 |
The temperature of the reversible heat source |
We may now make the following statements
 |
(First law of thermodynamics) |
 |
(Second law of thermodynamics) |
 |
(Reversible work source) |
 |
(Reversible heat source) |
Eliminating
 ,
,  , and
, and  gives the following equation:
gives the following equation:
When the primary system is reversible, the equality will hold and the amount of work delivered will be a maximum. Note that this will hold for any reversible system which has the same values of dU and dS .

