Surface diffusion
Encyclopedia
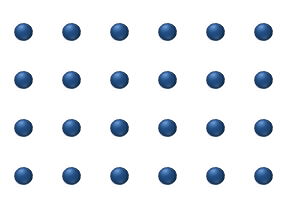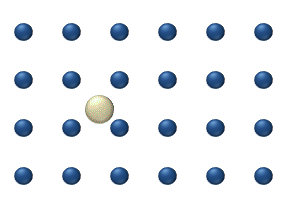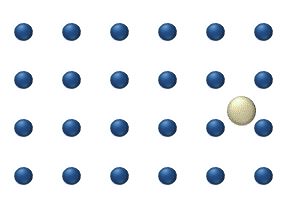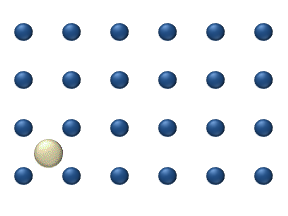
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s, and atomic clusters (adparticles) at solid material surface
Surface
In mathematics, specifically in topology, a surface is a two-dimensional topological manifold. The most familiar examples are those that arise as the boundaries of solid objects in ordinary three-dimensional Euclidean space R3 — for example, the surface of a ball...
s. The process can generally be thought of in terms of particles jumping between adjacent adsorption
Adsorption
Adsorption is the adhesion of atoms, ions, biomolecules or molecules of gas, liquid, or dissolved solids to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. It differs from absorption, in which a fluid permeates or is dissolved by a liquid or solid...
sites on a surface, as in figure 1. Just as in bulk diffusion
Diffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
, this motion is typically a thermally promoted process with rates increasing with increasing temperature. Many systems display diffusion behavior that deviates from the conventional model of nearest-neighbor jumps. Tunneling diffusion is a particularly interesting example of an unconventional mechanism wherein hydrogen has been shown to diffuse on clean metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
surfaces via the quantum tunneling effect.
Various analytical tools may be used to elucidate surface diffusion mechanisms and rates, the most important of which are field ion microscopy and scanning tunneling microscopy. While in principle the process can occur on a variety of materials, most experiments are performed on crystalline metal surfaces. Due to experimental constraints most studies of surface diffusion are limited to well below the melting point
Melting point
The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure...
of the substrate, and much has yet to be discovered regarding how these processes take place at higher temperatures.
Surface diffusion rates and mechanisms are affected by a variety of factors including the strength of the surface-adparticle bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
, orientation of the surface lattice, attraction and repulsion between surface species and chemical potential
Chemical potential
Chemical potential, symbolized by μ, is a measure first described by the American engineer, chemist and mathematical physicist Josiah Willard Gibbs. It is the potential that a substance has to produce in order to alter a system...
gradients. It is an important concept in surface phase formation
Phase transition
A phase transition is the transformation of a thermodynamic system from one phase or state of matter to another.A phase of a thermodynamic system and the states of matter have uniform physical properties....
, epitaxial growth, heterogeneous catalysis
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
, and other topics in surface science
Surface science
Surface science is the study of physical and chemical phenomena that occur at the interface of two phases, including solid–liquid interfaces, solid–gas interfaces, solid–vacuum interfaces, and liquid-gas interfaces. It includes the fields of surface chemistry and surface physics. Some related...
. As such, the principles of surface diffusion are critical for the chemical production
Chemical industry
The chemical industry comprises the companies that produce industrial chemicals. Central to the modern world economy, it converts raw materials into more than 70,000 different products.-Products:...
and semiconductor
Semiconductor
A semiconductor is a material with electrical conductivity due to electron flow intermediate in magnitude between that of a conductor and an insulator. This means a conductivity roughly in the range of 103 to 10−8 siemens per centimeter...
industries. Real-world applications relying heavily on these phenomena include catalytic converter
Catalytic converter
A catalytic converter is a device used to convert toxic exhaust emissions from an internal combustion engine into non-toxic substances. Inside a catalytic converter, a catalyst stimulates a chemical reaction in which noxious byproducts of combustion are converted to less toxic substances by dint...
s, integrated circuits used in electronic devices, and silver halide
Silver halide
A silver halide is one of the compounds formed between silver and one of the halogens — silver bromide , chloride , iodide , and three forms of silver fluorides. As a group, they are often referred to as the silver halides, and are often given the pseudo-chemical notation AgX...
salts used in photographic film
Photographic film
Photographic film is a sheet of plastic coated with an emulsion containing light-sensitive silver halide salts with variable crystal sizes that determine the sensitivity, contrast and resolution of the film...
.
Kinetics
Adsorption
Adsorption is the adhesion of atoms, ions, biomolecules or molecules of gas, liquid, or dissolved solids to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. It differs from absorption, in which a fluid permeates or is dissolved by a liquid or solid...
sites on a 2D lattice
Lattice (group)
In mathematics, especially in geometry and group theory, a lattice in Rn is a discrete subgroup of Rn which spans the real vector space Rn. Every lattice in Rn can be generated from a basis for the vector space by forming all linear combinations with integer coefficients...
, moving between adjacent (nearest-neighbor) adsorption sites by a jumping process. The jump rate is characterized by an attempt frequency
Frequency
Frequency is the number of occurrences of a repeating event per unit time. It is also referred to as temporal frequency.The period is the duration of one cycle in a repeating event, so the period is the reciprocal of the frequency...
and a thermodynamic factor that dictates the probability of an attempt resulting in a successful jump. The attempt frequency ν is typically taken to be simply the vibrational frequency
Molecular vibration
A molecular vibration occurs when atoms in a molecule are in periodic motion while the molecule as a whole has constant translational and rotational motion...
of the adatom, while the thermodynamic factor is a Boltzmann factor
Boltzmann factor
In physics, the Boltzmann factor is a weighting factor that determines the relative probability of a particle to be in a state i in a multi-state system in thermodynamic equilibrium at temperature T...
dependent on temperature and Ediff, the potential energy
Potential energy
In physics, potential energy is the energy stored in a body or in a system due to its position in a force field or due to its configuration. The SI unit of measure for energy and work is the Joule...
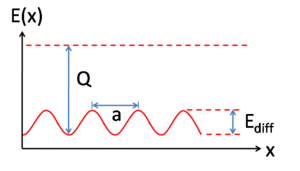
barrier to diffusion. Equation 1 describes the relationship:
Where ν and Ediff are as described above, Γ is the jump or hopping rate, T is temperature, and kB is the Boltzmann constant. Ediff must be smaller than the energy of desorption for diffusion to occur, otherwise desorption processes would dominate. Importantly, equation 1 tells us how very strongly the jump rate varies with temperature. The manner in which diffusion takes place is dependent on the relationship between Ediff and kBT as is given in the thermodynamic factor: when Ediff < kBT the thermodynamic factor approaches unity and Ediff ceases to be a meaningful barrier to diffusion. This case, known as mobile diffusion, is relatively uncommon and has only been observed in a few systems. For the phenomena described throughout this article, it is assumed that Ediff >> kBT and therefore Γ << ν. In the case of Fickian diffusion
Fick's law of diffusion
Fick's laws of diffusion describe diffusion and can be used to solve for the diffusion coefficient, D. They were derived by Adolf Fick in the year 1855.- Fick's first law :...
it is possible to extract both the ν and Ediff from an Arrhenius plot
Arrhenius plot
An Arrhenius plot displays the logarithm of kinetic constants plotted against inverse temperature . Arrhenius plots are often used to analyze the effect of temperature on the rates of chemical reactions...
of the logarithm of the diffusion coefficient, D, versus 1/T. For cases where more than one diffusion mechanism is present (see below), there may be more than one Ediff such that the relative distribution between the different processes would change with temperature.
Random walk
Random walk
A random walk, sometimes denoted RW, is a mathematical formalisation of a trajectory that consists of taking successive random steps. For example, the path traced by a molecule as it travels in a liquid or a gas, the search path of a foraging animal, the price of a fluctuating stock and the...
statistics describe the mean square displacement of diffusing species in terms of the number of jumps N and the distance per jump a. The number of successful jumps is simply Γ multiplied by the time allowed for diffusion, t. In the most basic model only nearest-neighbor jumps are considered and a corresponds to the spacing between nearest-neighbor adsorption sites. The root mean square displacement goes as
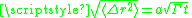 (eq. 2). The diffusion coefficient is given as D = Γa2/z (eq. 3), where z = 2 for 1D diffusion as would be the case for in-channel diffusion, z = 4 as in a square lattice
(eq. 2). The diffusion coefficient is given as D = Γa2/z (eq. 3), where z = 2 for 1D diffusion as would be the case for in-channel diffusion, z = 4 as in a square latticeSquare lattice
In mathematics, the square lattice is a type of lattice in a two-dimensional Euclidean space. It is the two-dimensional version of the integer lattice. It is one of the five types of two-dimensional lattices as classified by their symmetry groups; its symmetry group is known symbolically as p4m.Two...
, and z = 6 as in a hexagonal lattice
Hexagonal lattice
The hexagonal lattice or equilateral triangular lattice is one of the five 2D lattice types.Three nearby points form an equilateral triangle. In images four orientations of such a triangle are by far the most common...
.
Regimes
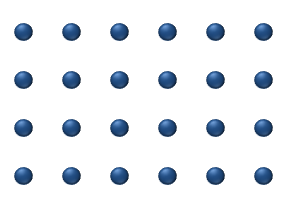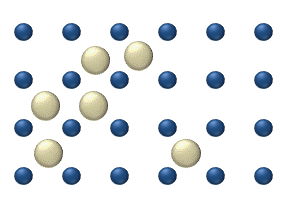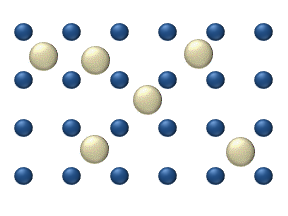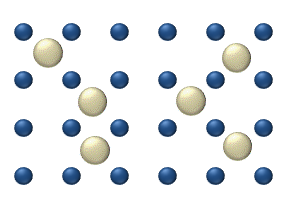
- Tracer diffusion describes the motion of individual adparticles on a surface at relatively low coverage levels. At these low levels (< 0.01 monolayerMonolayer- Chemistry :A Langmuir monolayer or insoluble monolayer is a one-molecule thick layer of an insoluble organic material spread onto an aqueous subphase. Traditional compounds used to prepare Langmuir monolayers are amphiphilic materials that possess a hydrophilic headgroup and a hydrophobic tail...
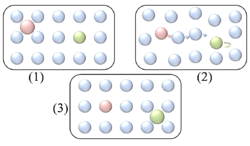
), particle interaction is low and each particle can be considered to move independently of the others. The single atom diffusing in figure 1 is a nice example of tracer diffusion. - Chemical diffusion describes the process at higher level of coverage where the effects of attraction or repulsion between adatoms becomes important. These interactions serve to alter the mobility of adatoms. In a crude way, figure 3 serves to show how adatoms may interact at higher coverage levels. The adatoms have no "choice" but to move to the right at first, and adjacent adatoms may block adsorption sites from one another.
- Intrinsic diffusion occurs on a uniform surface (e.g. lacking stepsTerrace ledge kinkThe Terrace Ledge Kink model, which is also referred to as the Terrace Step Kink model, describes the thermodynamics of crystal surface formation and transformation, as well as the energetics of surface defect formation...
or vacanciesTerrace ledge kinkThe Terrace Ledge Kink model, which is also referred to as the Terrace Step Kink model, describes the thermodynamics of crystal surface formation and transformation, as well as the energetics of surface defect formation...
) such as a single terrace, where no adatom traps or sources are present. This regime is often studied using field ion microscopy, wherein the terrace is a sharp sample tip on which an adparticle diffuses. Even in the case of a clean terrace the process may be influenced by non-uniformity near the edges of the terrace. - Mass transfer diffusion takes place in the case where adparticle sources and traps such as kinks, steps, and vacancies are present. Instead of being dependent only on the jump potential barrier Ediff, diffusion in this regime is now also dependent on the formation energy of mobile adparticles. The exact nature of the diffusion environment therefore plays a role in dictating the diffusion rate, since the formation energy of an adparticle is different for each type of surface feature as is described in the terrace-step-kink modelTerrace ledge kinkThe Terrace Ledge Kink model, which is also referred to as the Terrace Step Kink model, describes the thermodynamics of crystal surface formation and transformation, as well as the energetics of surface defect formation...
.
Anisotropy
Orientational anisotropy takes the form of a difference in both diffusion rates and mechanisms at the various surface orientationsMiller index
Miller indices form a notation system in crystallography for planes and directions in crystal lattices.In particular, a family of lattice planes is determined by three integers h, k, and ℓ, the Miller indices. They are written , and each index denotes a plane orthogonal to a direction in the...
of a given material. For a given crystalline material each Miller Index
Miller index
Miller indices form a notation system in crystallography for planes and directions in crystal lattices.In particular, a family of lattice planes is determined by three integers h, k, and ℓ, the Miller indices. They are written , and each index denotes a plane orthogonal to a direction in the...
plane may display unique diffusion phenomena. Close packed
Close-packing
In geometry, close-packing of equal spheres is a dense arrangement of congruent spheres in an infinite, regular arrangement . Carl Friedrich Gauss proved that the highest average density – that is, the greatest fraction of space occupied by spheres – that can be achieved by a regular lattice...
surfaces such as the fcc (111) tend to have higher diffusion rates than the correspondingly more "open" faces of the same material such as fcc (100).
Directional anistropy refers to a difference in diffusion mechanism or rate in a particular direction on a given crystallographic plane. These differences may be a result of either anisotropy in the surface lattice (e.g. a rectangular lattice
Lattice (group)
In mathematics, especially in geometry and group theory, a lattice in Rn is a discrete subgroup of Rn which spans the real vector space Rn. Every lattice in Rn can be generated from a basis for the vector space by forming all linear combinations with integer coefficients...
) or the presence of steps on a surface. One of the more dramatic examples of directional anistropy is the diffusion of adatoms on channeled surfaces such as fcc (110), where diffusion along the channel is much faster than diffusion across the channel.
Mechanisms
 |
 |
Adatom diffusion
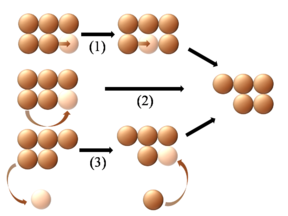
Diffusion of adatoms may occur by a variety of mechanisms. The manner in which they diffuse is important as it may dictate the kinetics of movement, temperature dependence, and overall mobility of surface species, among other parameters. The following is a summary of the most important of these processes:- Hopping or jumping is conceptually the most basic mechanism for diffusion of adatoms. In this model, the adatoms reside on adsorption sites on the surface lattice. Motion occurs through successive jumps to adjacent sites, the number of which depends on the nature of the surface lattice. Figures 1 and 3 both display adatoms undergoing diffusion via the hopping process. Studies have shown the presence of metastable transition states between adsorption sites wherein it may be possible for adatoms to temporarily reside.
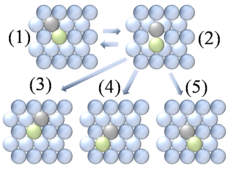
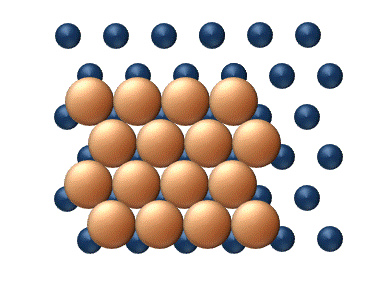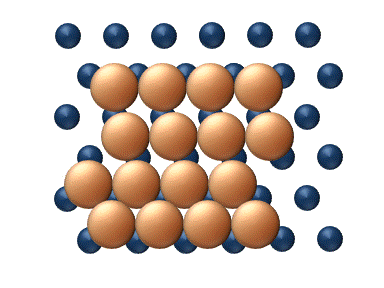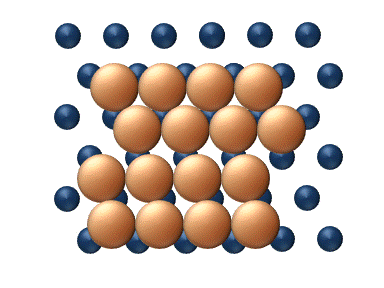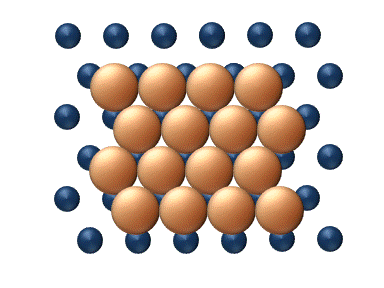
- Atomic exchange involves exchange between an adatom and an adjacent atom within the surface lattice. As shown in figure 4, after an atomic exchange event the adatom has taken the place of a surface atom and the surface atom has been displaced and has now become an adatom. This process may take place in both heterodiffusion (e.g. PtPlatinumPlatinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
adatoms on NiNickelNickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
) and self-diffusion (e.g. Pt adatoms on Pt). It is still unclear from a theoretical point of view why the atomic exchange mechanism is more predominant in some systems than in others. Current theory points towards multiple possibilities, including tensile surface stresses, surface relaxation about the adatom, and increased stability of the intermediate due to the fact that both atoms involved maintain high levels of coordinationCoordination numberIn chemistry and crystallography, the coordination number of a central atom in a molecule or crystal is the number of its nearest neighbours. This number is determined somewhat differently for molecules and for crystals....
throughout the process. - Tunneling diffusion is a physical manifestation of the quantum tunneling effect involving particles tunneling across diffusion barriers. It can occur in the case of low diffusing particle massMassMass can be defined as a quantitive measure of the resistance an object has to change in its velocity.In physics, mass commonly refers to any of the following three properties of matter, which have been shown experimentally to be equivalent:...
and low Ediff, and has been observed in the case of hydrogenHydrogenHydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
diffusion on tungstenTungstenTungsten , also known as wolfram , is a chemical element with the chemical symbol W and atomic number 74.A hard, rare metal under standard conditions when uncombined, tungsten is found naturally on Earth only in chemical compounds. It was identified as a new element in 1781, and first isolated as...
and copperCopperCopper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
surfaces. The phenomenon is unique in that in the regime where the tunneling mechanism dominates, the diffusion rate is nearly temperature-independent. - Vacancy diffusion can occur as the predominant method of surface diffusion at high coverage levels approaching complete coverage. This process is akin to the manner in which pieces slide around in a "sliding puzzleSliding puzzleA sliding puzzle, sliding block puzzle, or sliding tile puzzle is a puzzle that challenges a player to slide usually flat pieces along certain routes to establish a certain end-configuration....
". It is very difficult to directly observe vacancy diffusion due to the typically high diffusion rates and low vacancy concentrationConcentrationIn chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
. Figure 5 shows the basic theme of this mechanism in an albeit oversimplified manner.
 |
||
 |
 |
Recent theoretical work as well as experimental work performed since the late 1970s has brought to light a remarkable variety of surface diffusion phenomena both with regard to kinetics as well as to mechanisms. Following is a summary of some of the more notable phenomena:
- Long jumps consist of adatom displacement to a non-nearest-neighbor adsorption site. They may include double, triple, and longer jumps in the same direction as a nearest-neighbor jump would travel, or they may be in entirely different directions as shown in figure 6. They have been predicted by theoryTheoryThe English word theory was derived from a technical term in Ancient Greek philosophy. The word theoria, , meant "a looking at, viewing, beholding", and referring to contemplation or speculation, as opposed to action...
to exist in many different systems, and have been shown by experiment to take place at temperatures as low as 0.1 TmMelting pointThe melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure...
(melting temperature). In some cases data indicate long jumps dominating the diffusion process over single jumps at elevated temperatures; the phenomena of variable jump lengths is expressed in different characteristic distributions of atomic displacement over time (see figure 7). - Rebound jumps have been shown by both experiment and simulations to take place in certain systems. Since the motion does not result in a net displacement of the adatom involved, experimental evidence for rebound jumps again comes from statistical interpretation of atomic distributions. A rebound jump is shown in figure 6. The figure is slightly misleading, however, as rebound jumps have only been shown experimentally to take place in the case of 1D diffusion on a channeled surface (in particular, the bcc (211) face of tungsten).
- Cross-channel diffusion can occur in the case of channeled surfaces. Typically in-channel diffusion dominates due to the lower energy barrier for diffusion of this process. In certain cases cross-channel has been shown to occur, taking place in a manner similar to that shown in figure 8. The intermediate "dumbbell" position may lead to a variety of final adatom and surface atom displacements.
- Long-range atomic exchange is a process involving an adatom inserting into the surface as in the normal atomic exchange mechanism, but instead of a nearest-neighbor atom it is an atom some distance further from the initial adatom that emerges. Shown in figure 9, this process has only been observed in molecular dynamics simulations and has yet to be confirmed experimentally. In spite of this long range atomic exchange, as well as a variety of other exotic diffusion mechanisms, are anticipated to contribute substantially at temperatures currently too high for direct observation.
Cluster diffusion
Cluster diffusion involves motion of atomic clusters ranging in size from dimers to islands containing hundreds of atoms. Motion of the cluster may occur via the displacement of individual atoms, sections of the cluster, or the entire cluster moving at once. All of these processes involve a change in the cluster’s center of massCenter of mass
In physics, the center of mass or barycenter of a system is the average location of all of its mass. In the case of a rigid body, the position of the center of mass is fixed in relation to the body...
.
- Individual mechanisms are those that involve movement of one atom at a time.
- Edge diffusion involves movement of adatoms or vacancies at edge or kink sites. As shown in figure 10, the mobile atom maintains its proximity to the cluster throughout the process.
- Evaporation-condensation involves atoms “evaporatingEvaporationEvaporation is a type of vaporization of a liquid that occurs only on the surface of a liquid. The other type of vaporization is boiling, which, instead, occurs on the entire mass of the liquid....
” from the cluster onto a terrace accompanied by “condensationCondensationCondensation is the change of the physical state of matter from gaseous phase into liquid phase, and is the reverse of vaporization. When the transition happens from the gaseous phase into the solid phase directly, the change is called deposition....
” of terrace adatoms onto the cluster leading to a change in the cluster’s center of mass. While figure 10 appears to indicate the same atom evaporating from and condensing on the cluster, it may in fact be a different atom condensing from the 2D gas. - Leapfrog diffusion is similar to edge diffusion, but where the diffusing atom actually moves atop the cluster before settling in a different location from its starting position.
- Sequential displacement refers to the process involving motion one atom at a time, moving to free nearest-neighbor sites.

(a) Dislocation (b) Glide 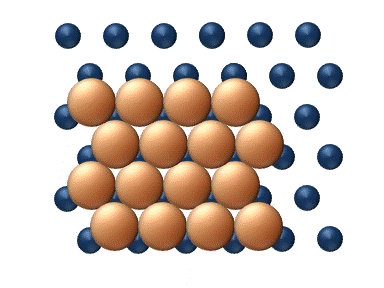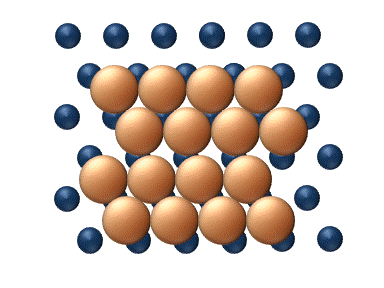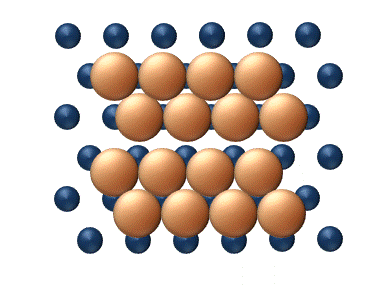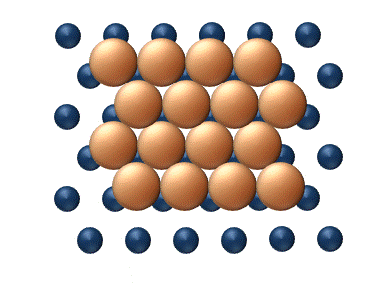
(c) Reptation (d) Shear Figure 11. Concerted mechanisms for cluster diffusion.
- Concerted mechanisms are those that involve movement of either sections of the cluster or the entire cluster all at once.
- Dislocation diffusion occurs when adjacent sub-units of a cluster move in a row-by-row fashion through displacement of a dislocationDislocationIn materials science, a dislocation is a crystallographic defect, or irregularity, within a crystal structure. The presence of dislocations strongly influences many of the properties of materials...
. As shown in figure 11(a) the process begins with nucleationNucleationNucleation is the extremely localized budding of a distinct thermodynamic phase. Some examples of phases that may form by way of nucleation in liquids are gaseous bubbles, crystals or glassy regions. Creation of liquid droplets in saturated vapor is also characterized by nucleation...
of the dislocation followed by what is essentially sequential displacement on a concerted basis. - Glide diffusion refers to the concerted motion of an entire cluster all at once (see figure 11(b)).
- Reptation is a snake-like movement (hence the name) involving sequential motion of cluster sub-units (see figure 11(c)).
- Shearing is a concerted displacement of a sub-unit of atoms within a cluster (see figure 11(d)).
- Dislocation diffusion occurs when adjacent sub-units of a cluster move in a row-by-row fashion through displacement of a dislocation
- Size-dependence: the rate of cluster diffusion has a strong dependence on the size of the cluster, with larger cluster size generally corresponding to slower diffusion. This is not, however, a universal trend and it has been shown in some systems that the diffusion rate takes on a periodic tendency wherein some larger clusters diffuse faster than those smaller than them.
Surface diffusion and heterogeneous catalysis
Surface diffusion is a critically important concept in heterogeneous catalysis, as reaction rates are often dictated by the ability of reactants to "find" each other at a catalyst surface. With increased temperature adsorbed molecules, molecular fragments, atoms, and clusters tend to have much greater mobility (see equation 1). However, with increased temperature the lifetime of adsorption decreases as the factor kBT becomes large enough for the adsorbed species to overcome the barrier to desorption, Q (see figure 2). ReactionChemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
thermodynamics
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
aside because of the interplay between increased rates of diffusion and decreased lifetime of adsorption, increased temperature may in some cases decrease the overall rate of the reaction.
Experimental
Surface diffusion may be studied by a variety of techniques, including both direct and indirect observations. Two experimental techniques that have proved very useful in this area of study are field ion microscopy and scanning tunneling microscopy. By visualizing the displacement of atoms or clusters over time, it is possible to extract useful information regarding the manner in which the relevant species diffuse-both mechanistic and rate-related information. In order to study surface diffusion on the atomistic scale it is unfortunately necessary to perform studies on rigorously clean surfaces and in UHVUHV
UHV may refer to:* University of Houston–Victoria* Ultra high vacuum* Ultra high voltage power line...
conditions or in the presence of small amounts of inert
Inert
-Chemistry:In chemistry, the term inert is used to describe a substance that is not chemically reactive.The noble gases were previously known as inert gases because of their perceived lack of participation in any chemical reactions...
gas, as is the case when using He or Ne as imaging gas in field-ion microscopy
Field ion microscope
Field ion microscopy is an analytical technique used in materials science. The field ion microscope is a type of microscope that can be used to image the arrangement of atoms at the surface of a sharp metal tip....
experiments.
Cited works
- G. Antczak, G. Ehrlich. Surface Science Reports 62 (2007), 39-61. (Review)