Transfer hydrogenation
Encyclopedia
Transfer hydrogenation is the addition of hydrogen
(H2; dihydrogen in inorganic
and organometallic
chemistry) to a molecule
from a source other than gaseous H2. It is applied in industry and in organic synthesis
, in part because of the inconvenience and expense of using gaseous H2. One large scale application of transfer hydrogenation is coal liquefaction
using "donor solvents" such as tetralin
.
, a useful family of hydrogen-transfer catalysts have been developed based on ruthenium
and rhodium
complexes with diamine
and phosphine
ligands. A representative catalyst precursor is derived from (cymene)ruthenium dichloride dimer
and the tosylated diphenylethylenediamine
. These catalysts are mainly employed for the reduction of ketones and imines to alcohols and amine
s, respectively. The hydrogen-donor (transfer agent) is typically isopropanol, which coverts to acetone
upon donation of hydrogen. Transfer hydrogenations can proceed with high enantioselectivities when the starting material is prochiral
:
where RR'C*H-OH is a chiral product. A typical catalyst is (cymene(R,R-HNCHPhCHPhNTs), where Ts = SO2C6H4Me
and R,R refers to the absolute configuration
of the two chiral carbon centers. This work was recognized with the 2001 Nobel Prize in Chemistry to Ryōji Noyori
. Another family of hydrogen-transfer agents are those based on aluminium alkoxides, such as Aluminium isopropoxide
.
 The diimide is generated from hydrazine
The diimide is generated from hydrazine
. Two hydrocarbons that can serve as hydrogen donors are cyclohexene
or cyclohexadiene
. In this case an alkane is formed along with the formation of benzene
. The driving force of the reaction being the gain of aromatic stabilization energy when benzene is formed. Pd can be used as a catalyst and a temperature of 100 °C is employed. One limitation of using transfer hydrogenation for the production of alkane is that it cannot be used to prepare methane as no unsaturated hydrocarbon contain only one carbon. More exotic transfer hydrogenations have been reported, including this intramolecular one:
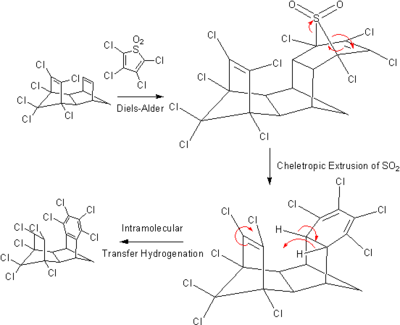 Many reactions exist with alcohol as the hydrogen donor. Examples are the sodium metal mediated Birch reduction
Many reactions exist with alcohol as the hydrogen donor. Examples are the sodium metal mediated Birch reduction
(arenes) and the Bouveault-Blanc reduction
(esters). The combination of magnesium
and methanol
is used in alkene reductions, e.g. the synthesis of asenapine
:
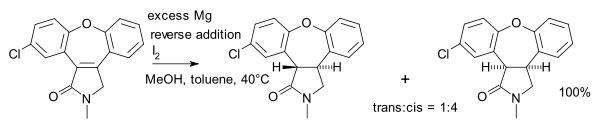
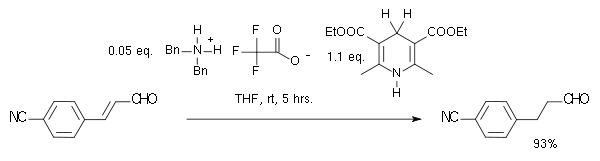 In this particular reaction the substrate is an α,β-unsaturated carbonyl compound. The proton donor is oxidized to the pyridine
In this particular reaction the substrate is an α,β-unsaturated carbonyl compound. The proton donor is oxidized to the pyridine
form and resembles the biochemically relevant coenzyme NADH. In the catalytic cycle
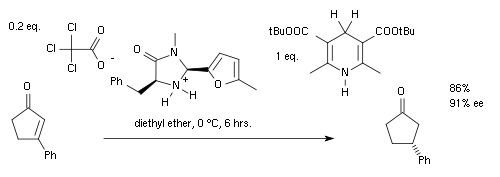
for this reaction the amine and the aldehyde first form an iminium ion, then proton transfer is followed by hydrolysis of the iminium bond regenerating the catalyst. By adopting a chiral imidazolidinone MacMillan organocatalyst an enantioselectivity of 81% ee
was obtained:
The group of MacMillan independently published a very similar asymmetric reaction in 2005 :
In an interesting case of stereoconvergence, both the E-isomer and the Z-isomer in this reaction yield the (S)-enantiomer
.
Extending the scope of this reaction towards ketone
s or rather enone
s requires fine tuning of the catalyst (add a benzyl
group and replace the t-butyl group by a furan
) and of the Hantzsch ester (add more bulky t-butyl groups) :
With a different organocatalyst altogether, hydrogenation can also be accomplished for imine
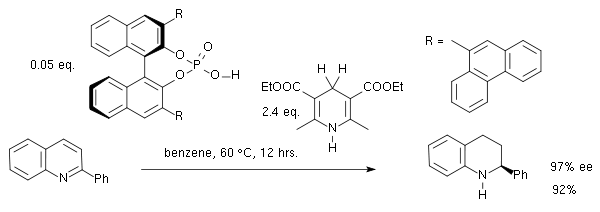
s. In one particular reaction the catalysts is a BINOL based phosphoric acid
, the substrate a quinoline
and the product a chiral tetradehydroquinoline in a 1,4-addition, isomerization and 1,2-addition cascade reaction
:
The first step in this reaction is protonation of the quinoline nitrogen atom by the phosphoric acid forming a transient chiral iminium ion. It is noted that with most traditional metal based catalysts, hydrogenation of aromatic or heteroaromatic substrates tend to fail.
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
(H2; dihydrogen in inorganic
Inorganic chemistry
Inorganic chemistry is the branch of chemistry concerned with the properties and behavior of inorganic compounds. This field covers all chemical compounds except the myriad organic compounds , which are the subjects of organic chemistry...
and organometallic
Organometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
chemistry) to a molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
from a source other than gaseous H2. It is applied in industry and in organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
, in part because of the inconvenience and expense of using gaseous H2. One large scale application of transfer hydrogenation is coal liquefaction
Coal liquefaction
-Methods:The liquefaction processes are classified as direct conversion to liquids processes and indirect conversion to liquids processeses. Direct processes are carbonization and hydrogenation.-Pyrolysis and carbonization processes:...
using "donor solvents" such as tetralin
Tetralin
Tetralin is a hydrocarbon having the chemical formula C10H12. This molecule is similar to the naphthalene chemical structure except that one ring is saturated.The compound can be synthesized in a Bergman cyclization...
.
Organometallic catalysts
In the area of organic synthesisOrganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
, a useful family of hydrogen-transfer catalysts have been developed based on ruthenium
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
and rhodium
Rhodium
Rhodium is a chemical element that is a rare, silvery-white, hard and chemically inert transition metal and a member of the platinum group. It has the chemical symbol Rh and atomic number 45. It is composed of only one isotope, 103Rh. Naturally occurring rhodium is found as the free metal, alloyed...
complexes with diamine
Diamine
A diamine is a type of polyamine with exactly two amino groups. Diamines are mainly used as monomers to prepare polyamides, polyimides and polyureas. In terms of quantities produced, 1,6-diaminohexane, a precursor to Nylon 6-6, is most important, followed by ethylenediamine...
and phosphine
Phosphine
Phosphine is the compound with the chemical formula PH3. It is a colorless, flammable, toxic gas. Pure phosphine is odourless, but technical grade samples have a highly unpleasant odor like garlic or rotting fish, due to the presence of substituted phosphine and diphosphine...
ligands. A representative catalyst precursor is derived from (cymene)ruthenium dichloride dimer
(Cymene)ruthenium dichloride dimer
ruthenium dichloride dimer is the organometallic compound with the formula [RuCl2]2. This red-coloured, diamagnetic solid is a reagent in organometallic chemistry and homogeneous catalysis.-Preparation and reactions:...
and the tosylated diphenylethylenediamine
Diphenylethylenediamine
1,2-Diphenyl-1,2-ethylenediamine is an organic compound with the formula H2NCHPhCHPhNH2, where Ph is C6H5, phenyl. This diamine is a precursor to a ligand for certain homogeneous hydrogenation catalysts...
. These catalysts are mainly employed for the reduction of ketones and imines to alcohols and amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s, respectively. The hydrogen-donor (transfer agent) is typically isopropanol, which coverts to acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
upon donation of hydrogen. Transfer hydrogenations can proceed with high enantioselectivities when the starting material is prochiral
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
:
- RR'C=O + Me2CHOH → RR'C*H-OH + Me2C=O
where RR'C*H-OH is a chiral product. A typical catalyst is (cymene(R,R-HNCHPhCHPhNTs), where Ts = SO2C6H4Me
Tosyl
A tosyl group is CH3C6H4SO2. This group is usually derived from the compound 4-toluenesulfonyl chloride, CH3C6H4SO2Cl, which forms esters and amides of toluenesulfonic or tosylic acid...
and R,R refers to the absolute configuration
Absolute configuration
An absolute configuration in stereochemistry is the spatial arrangement of the atoms of a chiral molecular entity and its stereochemical description e.g. R or S....
of the two chiral carbon centers. This work was recognized with the 2001 Nobel Prize in Chemistry to Ryōji Noyori
Ryoji Noyori
is a Japanese chemist. He won the Nobel Prize in Chemistry in 2001. Noyori shared half of the prize with William S. Knowles for the study of chirally catalyzed hydrogenations; the second half of the Prize went to K. Barry Sharpless for his study in chirally catalyzed oxidation reactions...
. Another family of hydrogen-transfer agents are those based on aluminium alkoxides, such as Aluminium isopropoxide
Aluminium isopropoxide
Aluminium isopropoxide is the chemical compound usually described with the formula Al3, where i-Pr is the isopropyl group . This colourless solid is a useful reagent in organic synthesis...
.
Hydrogen donors
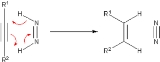
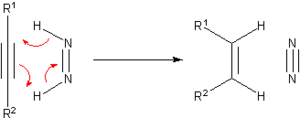
A historically prominent transfer hydrogenation agent is diimide, which becomes oxidized to the very stable N2:
Hydrazine
Hydrazine is an inorganic compound with the formula N2H4. It is a colourless flammable liquid with an ammonia-like odor. Hydrazine is highly toxic and dangerously unstable unless handled in solution. Approximately 260,000 tons are manufactured annually...
. Two hydrocarbons that can serve as hydrogen donors are cyclohexene
Cyclohexene
Cyclohexene is a hydrocarbon with the formula C6H10. This cycloalkene is a colorless liquid with a sharp smell. It is an intermediate in various industrial processes...
or cyclohexadiene
Cyclohexadiene
-See also:* Benzene or its theoretical isomer 1,3,5-Cyclohexatriene* Cyclohexene...
. In this case an alkane is formed along with the formation of benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
. The driving force of the reaction being the gain of aromatic stabilization energy when benzene is formed. Pd can be used as a catalyst and a temperature of 100 °C is employed. One limitation of using transfer hydrogenation for the production of alkane is that it cannot be used to prepare methane as no unsaturated hydrocarbon contain only one carbon. More exotic transfer hydrogenations have been reported, including this intramolecular one:

Birch reduction
The Birch Reduction is an organic reaction which is particularly useful in synthetic organic chemistry. The reaction was reported in 1944 by the Australian chemist Arthur Birch working in the Dyson Perrins Laboratory in the University of Oxford, building on earlier work by Wooster and Godfrey in...
(arenes) and the Bouveault-Blanc reduction
Bouveault-Blanc reduction
The Bouveault-Blanc reduction is a chemical reaction in which an ester is reduced to primary alcohols using absolute ethanol and sodium metal.This reaction is an inexpensive and large-scale alternative to lithium aluminium hydride reduction of esters....
(esters). The combination of magnesium
Magnesium
Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole...
and methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
is used in alkene reductions, e.g. the synthesis of asenapine
Asenapine
Asenapine is a new atypical antipsychotic developed for the treatment of schizophrenia and acute mania associated with bipolar disorder by Schering-Plough after its November 19, 2007 merger with Organon International. Development of the drug, through Phase III trials, began while Organon was still...
:

Organocatalytic transfer hydrogenation
Organocatalytic transfer hydrogenation has been described by the group of List in 2004 in a system with a Hantzsch ester as proton donor and an amine catalyst:
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
form and resembles the biochemically relevant coenzyme NADH. In the catalytic cycle
Catalytic cycle
A catalytic cycle in chemistry is a term for a multistep reaction mechanism that involves a catalyst . The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, materials science, etc. Often such cycles show the conversion of a...
for this reaction the amine and the aldehyde first form an iminium ion, then proton transfer is followed by hydrolysis of the iminium bond regenerating the catalyst. By adopting a chiral imidazolidinone MacMillan organocatalyst an enantioselectivity of 81% ee
Enantiomeric excess
The enantiomeric excess of a substance is a measure of how pure it is. In this case, the impurity is the undesired enantiomer .-Definition:...
was obtained:
The group of MacMillan independently published a very similar asymmetric reaction in 2005 :
In an interesting case of stereoconvergence, both the E-isomer and the Z-isomer in this reaction yield the (S)-enantiomer
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
.
Extending the scope of this reaction towards ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s or rather enone
Enone
An enone is an unsaturated chemical compound or functional group consisting of a conjugated system of an alkene and a ketone. The simplest enone is methyl vinyl ketone or CH2=CHCOCH3....
s requires fine tuning of the catalyst (add a benzyl
Benzyl
In organic chemistry, benzyl is the term used to describe the substituent or molecular fragment possessing the structure C6H5CH2-. Benzyl features a benzene ring attached to a CH2 group.-Nomenclature:...
group and replace the t-butyl group by a furan
Furan
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen. The class of compounds containing such rings are also referred to as furans....
) and of the Hantzsch ester (add more bulky t-butyl groups) :
With a different organocatalyst altogether, hydrogenation can also be accomplished for imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
s. In one particular reaction the catalysts is a BINOL based phosphoric acid
Phosphoric acid
Phosphoric acid, also known as orthophosphoric acid or phosphoric acid, is a mineral acid having the chemical formula H3PO4. Orthophosphoric acid molecules can combine with themselves to form a variety of compounds which are also referred to as phosphoric acids, but in a more general way...
, the substrate a quinoline
Quinoline
Quinoline is a heterocyclic aromatic organic compound. It has the formula C9H7N and is a colourless hygroscopic liquid with a strong odour. Aged samples, if exposed to light, become yellow and later brown...
and the product a chiral tetradehydroquinoline in a 1,4-addition, isomerization and 1,2-addition cascade reaction
Cascade reaction
A cascade reaction or tandem reaction or domino reaction is a consecutive series of intramolecular organic reactions which often proceed via highly reactive intermediates. It allows the organic synthesis of complex multinuclear molecules from a single acyclic precursor. The substrate contains many...
:
The first step in this reaction is protonation of the quinoline nitrogen atom by the phosphoric acid forming a transient chiral iminium ion. It is noted that with most traditional metal based catalysts, hydrogenation of aromatic or heteroaromatic substrates tend to fail.
See also
- Meerwein–Ponndorf–Verley reduction
- Oppenauer oxidationOppenauer oxidationOppenauer oxidation, named after Rupert Viktor Oppenauer, is a gentle method for selectively oxidizing secondary alcohols to ketones.The reaction is the opposite of Meerwein-Ponndorf-Verley reduction. The alcohol is oxidized with aluminium isopropoxide in excess acetone...
- DehydrogenationDehydrogenationDehydrogenation is a chemical reaction that involves the elimination of hydrogen . It is the reverse process of hydrogenation. Dehydrogenation reactions may be either large scale industrial processes or smaller scale laboratory procedures....
- HydrogenationHydrogenationHydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
- HydrogenolysisHydrogenolysisHydrogenolysis is a chemical reaction whereby a carbon–carbon or carbon–heteroatom single bond is cleaved or undergoes "lysis" by hydrogen. The heteroatom may vary, but it usually is oxygen, nitrogen, or sulfur. A related reaction is hydrogenation, where hydrogen is added to the molecule, without...