
Coordination sphere
Encyclopedia
.png)
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s, around.
Molecules that are attached noncovalently to the ligands are called the second coordination sphere.
Second coordination sphere
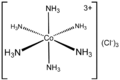
The second coordination sphere refers to the molecules that are attached noncovalently to ligands that occupy the first coordination sphere. These molecules are typically solvent. The interactions between the first and second coordination spheres usually involve hydrogen-bonding. For charged complexes, ion-pairing is important. Solvent effects are pronounced in complexes where the ligands in the first coordination sphere are strong hydrogen-bond donors and acceptors, e.g. respectively [Co(NH3)6]3+ and [Fe(CN)6]3-Ferricyanide
Ferricyanide is the anion [Fe6]3−. It is also called hexacyanoferrate and in rare, but systematic nomenclature, hexacyanidoferrate...
. Crown-ethers bind to polyamine complexes through their second coordination sphere.
Example
In biological and computational chemistry
Mechanisms of metalloproteinMetalloprotein
Metalloprotein is a generic term for a protein that contains a metal ion cofactor. Metalloproteins have many different functions in cells, such as enzymes, transport and storage proteins, and signal transduction proteins. Indeed, about one quarter to one third of all proteins require metals to...
s often invoke modulation of the second coordination sphere by the protein. In solution, metal ions can be described as consisting of series of two concentric coordination spheres, the first and second. More distant from the second coordination sphere, the solvent molecules behave more like "bulk solvent." Simulation of this solvation
Solvation
Solvation, also sometimes called dissolution, is the process of attraction and association of molecules of a solvent with molecules or ions of a solute...
is of interest in computational chemistry
Computational chemistry
Computational chemistry is a branch of chemistry that uses principles of computer science to assist in solving chemical problems. It uses the results of theoretical chemistry, incorporated into efficient computer programs, to calculate the structures and properties of molecules and solids...
.
Role in mechanistic inorganic chemistry
The rates at which ligands exchange between the first and the second coordination sphere is the first step in ligand substitution reactions. In associative ligand substitutionAssociative substitution
Associative substitution describes a pathway by which compounds interchange ligands. The terminology is typically applied to coordination and organometallic complexes, but resembles the Sn2 mechanism in organic chemistry. The opposite pathway is dissociative substitution, being analogous to Sn1...
, the entering nucleophile enters via the second coordination sphere. These rates vary over many orders of magnitude. The energetics of many inner sphere electron transfer
Inner sphere electron transfer
Inner sphere or bonded electron transfer proceeds via a covalent linkage between the two redox partners, the oxidant and the reductant. In Inner Sphere electron transfer , a ligand bridges the two metal redox centers during the electron transfer event. Inner sphere reactions are inhibited by...
reactions are discussed in terms of second coordination sphere. Some proton coupled electron transfer
Proton coupled electron transfer
Proton-coupled electron transfer is a reaction mechanism that is thought to be common in redox reactions. It involves the concerted transfer of an electron and proton to or from a substrate....
reactions involve atom transfer between the second coordination spheres of the reactants:
- [Fe*(H2O)6]2+ + [Fe(H2O)5(OH)]2+ → [Fe(H2O)6]3+ + [Fe*(H2O)5(OH)]2+

