
Haloalkane
Encyclopedia

Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
s derived from alkane
Alkane
Alkanes are chemical compounds that consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds without any cycles...
s containing one or more halogen
Halogen
The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
s. They are a subset of the general class of halocarbon
Halocarbon
Halocarbon compounds are chemicals in which one or more carbon atoms are linked by covalent bonds with one or more halogen atoms resulting in the formation of organofluorine compounds, organochlorine compounds, organobromine compounds, and organoiodine compounds...
s, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants
Fire extinguisher
A fire extinguisher or extinguisher, flame entinguisher is an active fire protection device used to extinguish or control small fires, often in emergency situations...
, refrigerant
Refrigerant
A refrigerant is a substance used in a heat cycle usually including, for enhanced efficiency, a reversible phase change from a liquid to a gas. Traditionally, fluorocarbons, especially chlorofluorocarbons, were used as refrigerants, but they are being phased out because of their ozone depletion...
s, propellant
Propellant
A propellant is a material that produces pressurized gas that:* can be directed through a nozzle, thereby producing thrust ;...
s, solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
s, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutant
Pollutant
A pollutant is a waste material that pollutes air, water or soil, and is the cause of pollution.Three factors determine the severity of a pollutant: its chemical nature, its concentration and its persistence. Some pollutants are biodegradable and therefore will not persist in the environment in the...
s and toxins. For example, the chlorofluorocarbon
Chlorofluorocarbon
A chlorofluorocarbon is an organic compound that contains carbon, chlorine, and fluorine, produced as a volatile derivative of methane and ethane. A common subclass are the hydrochlorofluorocarbons , which contain hydrogen, as well. They are also commonly known by the DuPont trade name Freon...
s have been shown to lead to ozone depletion
Ozone depletion
Ozone depletion describes two distinct but related phenomena observed since the late 1970s: a steady decline of about 4% per decade in the total volume of ozone in Earth's stratosphere , and a much larger springtime decrease in stratospheric ozone over Earth's polar regions. The latter phenomenon...
. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gas
Greenhouse gas
A greenhouse gas is a gas in an atmosphere that absorbs and emits radiation within the thermal infrared range. This process is the fundamental cause of the greenhouse effect. The primary greenhouse gases in the Earth's atmosphere are water vapor, carbon dioxide, methane, nitrous oxide, and ozone...
es. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halon
Halon
Halon can refer to:* Haloalkane, or halogenoalkane, a group of chemical compounds consisting of alkanes with linked halogens. In particular, bromine-containing haloalkanes.* Halomethane fire extinguishing systems...
.
Haloalkanes have been known for centuries. Ethyl chloride was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation
Hydrohalogenation
A hydrohalogenation reaction is the electrophilic addition of hydrohalic acids like hydrogen chloride or hydrogen bromide to alkenes to yield the corresponding haloalkanes....
of alkenes, and the conversion of alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.
While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically-produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols).
Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.
Classes of haloalkanes
From the structural perspective, haloalkanes can be classified according to the connectivity of the carbon atom to which the halogen is attached. In primary (1°) haloalkanes, the carbonCarbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
that carries the halogen atom is only attached to one other alkyl group. An example is chloroethane
Chloroethane
Chloroethane or monochloroethane, commonly known by its old name ethyl chloride, is a chemical compound with chemical formula , once widely used in producing tetraethyllead, a gasoline additive...
. In secondary (2°) haloalkanes, the carbon that carries the halogen atom has two C–C bonds. In tertiary (3°) haloalkanes, the carbon that carries the halogen atom has three C–C bonds.
Haloalkanes can also be classified according to the type of halogen. Haloalkanes containing carbon bonded to fluorine
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
, chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
, bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
, and iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
results in organofluorine
Organofluorine chemistry
Organofluorine chemistry describes the chemistry of organofluorine compounds, organic compounds that contain the carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil- and water-repellents to pharmaceuticals, refrigerants and reagents in catalysis...
, organochlorine
Organochloride
An organochloride, organochlorine, chlorocarbon, chlorinated hydrocarbon, or chlorinated solvent is an organic compound containing at least one covalently bonded chlorine atom. Their wide structural variety and divergent chemical properties lead to a broad range of applications...
, organobromine
Organobromine compound
Organobromine compounds are organic compounds that contain carbon bonded to bromine. The most pervasive is the naturally produced bromomethane. One prominent application is the use of polybrominated diphenyl ethers as fire-retardants. A variety of minor organobromine compounds are found in...
and organoiodine
Organoiodine compound
Organoiodine compounds are organic compounds that contain one or more carbon–iodine bonds. They occur widely in organic chemistry, but are relatively rare in nature...
compounds, respectively. Compounds containing more than one kind of halogen are also possible. Several classes of widely used haloalkanes are classified in this way chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HClFCs) and hydrofluorocarbons (HFCs). These abbreviations are particularly common in discussions of the environmental impact of haloalkanes.
Properties
Haloalkanes generally resemble the parent alkanes in being colorless, relatively odorless, and hydrophobic. Their boiling points are higher than the corresponding alkanes and scale with the atomic weight and number of halides. This is due to the increased strength of the intermolecular forceIntermolecular force
Intermolecular forces are forces of attraction or repulsion which act between neighboring particles: atoms, molecules or ions. They are weak compared to the intramolecular forces, the forces which keep a molecule together...
s—from London dispersion
London dispersion force
London dispersion forces is a type of force acting between atoms and molecules. They are part of the van der Waals forces...
to dipole-dipole interaction because of the increased polarity. Thus carbon tetraiodide
Carbon tetraiodide
Carbon tetraiodide is CI4, a tetrahalomethane. Being bright red, it is a relatively rare example of a highly colored methane derivative. It is only 2% by weight carbon, although other methane derivatives are known with still less carbon....
is a solid whereas carbon tetrafluoride
Tetrafluoromethane
Tetrafluoromethane, also known as carbon tetrafluoride, is the simplest fluorocarbon . It has a very high bond strength due to the nature of the carbon–fluorine bond. It can also be classified as a haloalkane or halomethane...
is a gas. As they contain fewer C–H bonds, halocarbons are less flammable than alkanes, and some are used in fire extinguishers. Haloalkanes are better solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
s than the corresponding alkanes because of their increased polarity. Haloalkanes containing halogens other than fluorine are more reactive than the parent alkanes—it is this reactivity that is the basis of most controversies. Many are alkylating agents, with primary haloalkanes and those containing heavier halogens being the most active (fluoroalkanes do not act as alkylating agents under normal conditions). The ozone-depleting abilities of the CFCs arises from the photolability of the C–Cl bond.
Occurrence
Haloalkanes are of wide interest because they are widespread and have diverse beneficial and detrimental impacts. The oceans are estimated to release 1-2 million tons of bromomethane annually.A large number of pharmaceuticals contain halogens, especially fluorine
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
. An estimated one fifth of pharmaceuticals contain fluorine, including several of the top drugs. Examples include 5-fluorouracil, fluoxetine
Fluoxetine
Fluoxetine is an antidepressant of the selective serotonin reuptake inhibitor class. It is manufactured and marketed by Eli Lilly and Company...
(Prozac), paroxetine
Paroxetine
Paroxetine is an SSRI antidepressant. Marketing of the drug began in 1992 by the pharmaceutical company SmithKline Beecham, now GlaxoSmithKline...
(Paxil), ciprofloxacin
Ciprofloxacin
Ciprofloxacin is a synthetic chemotherapeutic antibiotic of the fluoroquinolone drug class.It is a second-generation fluoroquinolone antibacterial. It kills bacteria by interfering with the enzymes that cause DNA to rewind after being copied, which stops synthesis of DNA and of...
(Cipro), mefloquine
Mefloquine
Mefloquine hydrochloride is an orally administered medication used in the prevention and treatment of malaria. Mefloquine was developed in the 1970s at the United States Department of Defense's Walter Reed Army Institute of Research as a synthetic analogue of quinine...
, and fluconazole
Fluconazole
Fluconazole is a triazole antifungal drug used in the treatment and prevention of superficial and systemic fungal infections. In a bulk powder form, it appears as a white crystalline powder, and it is very slightly soluble in water and soluble in alcohol. It is commonly marketed under the trade...
. The beneficial effects arise because the C-F bond is relatively unreactive. Fluorine-substituted ethers are volatile anesthetics, including the commercial products methoxyflurane
Methoxyflurane
Methoxyflurane is a halogenated ether that was in clinical use as an volatile inhalational anesthetic from its introduction by Joseph F. Artusio et al in 1960 until around 1974. It was first synthesized in the late 1940s by William T...
, enflurane
Enflurane
Enflurane is a halogenated ether that was commonly used for inhalational anesthesia during the 1970s and 1980s. Developed by Ross Terrell in 1963, it was first used clinically in 1966....
, isoflurane
Isoflurane
Isoflurane is a halogenated ether used for inhalational anesthesia. Together with enflurane and halothane, it replaced the flammable ethers used in the pioneer days of surgery. Its name comes from being a structural isomer of enflurane, hence they have the same empirical formula...
, sevoflurane
Sevoflurane
Sevoflurane , also called fluoromethyl hexafluoroisopropyl ether, is a sweet-smelling, nonflammable, highly fluorinated methyl isopropyl ether used for induction and maintenance of general anesthesia. Together with desflurane, it is replacing isoflurane and halothane in modern anesthesiology...
and desflurane
Desflurane
Desflurane is a highly fluorinated methyl ethyl ether used for maintenance of general anesthesia. Like halothane, enflurane and isoflurane, it is a racemic mixture of and optical isomers...
. Fluorocarbon anesthetics reduce the hazard of flammability with diethyl ether
Diethyl ether
Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
and cyclopropane
Cyclopropane
Cyclopropane is a cycloalkane molecule with the molecular formula C3H6, consisting of three carbon atoms linked to each other to form a ring, with each carbon atom bearing two hydrogen atoms...
. Perfluorinated alkanes are used as blood substitute
Blood substitute
A blood substitute is a substance used to mimic and fulfill some functions of biological blood, usually in the oxygen-carrying sense...
s.
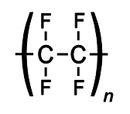
Polyvinyl chloride
Polyvinyl chloride, commonly abbreviated PVC, is a thermoplastic polymer. It is a vinyl polymer constructed of repeating vinyl groups having one hydrogen replaced by chloride. Polyvinyl chloride is the third most widely produced plastic, after polyethylene and polypropylene. PVC is widely used in...
(PVC), and polytetrafluoroethene (PTFE, or Teflon). The production of these materials releases substantial amounts of wastes.
IUPAC
The formal naming of haloalkanes should follow IUPAC nomenclatureIUPAC nomenclature
A chemical nomenclature is a set of rules to generate systematic names for chemical compounds. The nomenclature used most frequently worldwide is the one created and developed by the International Union of Pure and Applied Chemistry ....
, which put the halogen as a prefix to the alkane. For example, ethane
Ethane
Ethane is a chemical compound with chemical formula C2H6. It is the only two-carbon alkane that is an aliphatic hydrocarbon. At standard temperature and pressure, ethane is a colorless, odorless gas....
with bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
becomes bromoethane
Bromoethane
Bromoethane, also known as ethyl bromide, is a chemical compound of the haloalkanes group. It is abbreviated by chemists as EtBr. This volatile compound has an ether-like odour.-Preparation:...
, methane
Methane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
with four chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
groups becomes tetrachloromethane
Carbon tetrachloride
Carbon tetrachloride, also known by many other names is the organic compound with the formula CCl4. It was formerly widely used in fire extinguishers, as a precursor to refrigerants, and as a cleaning agent...
. However, many of these compounds have already an established trivial name, which is endorsed by the IUPAC nomenclature, for example chloroform
Chloroform
Chloroform is an organic compound with formula CHCl3. It is one of the four chloromethanes. The colorless, sweet-smelling, dense liquid is a trihalomethane, and is considered somewhat hazardous...
(trichloromethane) and methylene chloride (dichloromethane
Dichloromethane
Dichloromethane is an organic compound with the formula CH2Cl2. This colorless, volatile liquid with a moderately sweet aroma is widely used as a solvent. Although it is not miscible with water, it is miscible with many organic solvents...
). For unambiguity, this article follows the systematic naming scheme throughout.
Production
Haloalkanes can be produced from virtually all organic precursors. From the perspective of industry, the most important ones are alkanes and alkenes.From alkanes
Alkanes react with halogens by free radical halogenationFree radical halogenation
In organic chemistry, free-radical halogenation is a type of halogenation. This chemical reaction is typical of alkanes and alkyl-substituted aromatics under application of heat or UV light. The reaction is used for the industrial synthesis of chloroform , dichloromethane , and hexachlorobutadiene...
. In this reaction a hydrogen atom is removed from the alkane, then replaced by a halogen atom by reaction with a diatomic halogen molecule. The reactive intermediate
Reactive intermediate
In chemistry a reactive intermediate is a short-lived, high energy, highly reactive molecule. When generated in a chemical reaction it will quickly convert into a more stable molecule. Only in exceptional cases can these compounds be isolated and stored, e.g. low temperatures, matrix isolation...
in this reaction is a free radical and the reaction is called a radical chain reaction.
Free radical halogenation typically produces a mixture of compounds mono- or multihalogenated at various positions. It is possible to predict the results of a halogenation reaction based on bond dissociation energies and the relative stabilities of the radical intermediates. Another factor to consider is the probability of reaction at each carbon atom, from a statistical point of view.
Due to the different dipole moments of the product mixture, it may be possible to separate them by distillation
Distillation
Distillation is a method of separating mixtures based on differences in volatilities of components in a boiling liquid mixture. Distillation is a unit operation, or a physical separation process, and not a chemical reaction....
.
From alkenes and alkynes
In hydrohalogenationHydrohalogenation
A hydrohalogenation reaction is the electrophilic addition of hydrohalic acids like hydrogen chloride or hydrogen bromide to alkenes to yield the corresponding haloalkanes....
, an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
reacts with a dry hydrogen halide (HX) like hydrogen chloride
Hydrogen chloride
The compound hydrogen chloride has the formula HCl. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric humidity. Hydrogen chloride gas and hydrochloric acid are important in technology and industry...
or hydrogen bromide
Hydrogen bromide
Hydrogen bromide is the diatomic molecule HBr. HBr is a gas at standard conditions. Hydrobromic acid forms upon dissolving HBr in water. Conversely, HBr can be liberated from hydrobromic acid solutions with the addition of a dehydration agent, but not by distillation. Hydrogen bromide and...
to form a mono-haloalkane. The double bond of the alkene is replaced by two new bonds, one with the halogen and one with the hydrogen atom of the hydrohalic acid. Markovnikov's rule
Markovnikov's rule
In organic chemistry, Markovnikov's rule or Markownikoff's rule is an observation based on Zaitsev's rule. It was formulated by the Russian chemist Vladimir Vasilevich Markovnikov in 1870....
states that in this reaction, the halogen is more likely to become attached to the more substituted carbon. This is a electrophilic addition
Electrophilic addition
In organic chemistry, an electrophilic addition reaction is an addition reaction where, in a chemical compound, a π bond is broken and two new σ bonds are formed...
reaction. Water must be absent otherwise there will be a side product of a halohydrin
Halohydrin
A halohydrin or a haloalcohol is a type of organic compound or functional group in which one carbon atom has a halogen substituent, and an adjacent carbon atom has a hydroxyl substituent. They are derived from alcohols are therefore characterized by the presence of both the hydroxyl functional...
. The reaction is necessarily to be carried out in a dry inert solvent such as or directly in the gaseous phase. The reaction of alkynes are similar, with the product being a geminal dihalide; once again, Markovnikov's rule
Markovnikov's rule
In organic chemistry, Markovnikov's rule or Markownikoff's rule is an observation based on Zaitsev's rule. It was formulated by the Russian chemist Vladimir Vasilevich Markovnikov in 1870....
is followed.
Alkenes also react with halogens (X2) to form haloalkanes with two neighboring halogen atoms in a halogen addition reaction
Halogen addition reaction
A halogen addition reaction is a simple organic reaction where a halogen molecule is added to the carbon–carbon double bond of an alkene functional group.The general chemical formula of the halogen addition reaction is:...
. Alkynes react similarly, forming the tetrahalo compounds. This is sometimes known as "decolorizing" the halogen, since the reagent X2 is colored and the product is usually colorless.
From alcohols
Tertiary alkanol reacts with hydrochloric acidHydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
directly to produce tertiary chloroalkane, but if primary or secondary alkanol is used, an activator such as zinc chloride
Zinc chloride
Zinc chloride is the name of chemical compound with the formula ZnCl2 and its hydrates. Zinc chlorides, of which nine crystalline forms are known, are colorless or white, and are highly soluble in water. ZnCl2 itself is hygroscopic and even deliquescent. Samples should therefore be protected from...
is needed. This reaction is exploited in the Lucas test
Lucas test
Lucas test may refer to* The Lucas primality test for primality of general numbers* The Lucas–Lehmer primality test for Mersenne primes* Lucas' reagent, used to classify alcohols of low molecular weight...
.
The most popular conversion is effected by reacting the alcohol with thionyl chloride
Thionyl chloride
Thionyl chloride is an inorganic compound with the formula SOCl2. It is a reactive chemical reagent used in chlorination reactions. It is a colorless, distillable liquid at room temperature and pressure that decomposes above 140 °C. Thionyl chloride is sometimes confused with sulfuryl...
in the "Darzen's Process," which is one of the most convenient laboratory methods because the byproducts are gaseous. Both phosphorus pentachloride and phosphorus trichloride
Phosphorus trichloride
Phosphorus trichloride is a chemical compound of phosphorus and chlorine, having chemical formula PCl3. Its shape is trigonal pyramidal. It is the most important of the three phosphorus chlorides. It is an important industrial chemical, being used for the manufacture of organophosphorus compounds...
also convert the hydroxyl group to the chloride.
Alcohols may likewise be converted to bromoalkanes using hydrobromic acid
Hydrobromic acid
Hydrobromic acid is a strong acid formed by dissolving the diatomic molecule hydrogen bromide in water. "Constant boiling" hydrobromic acid is an aqueous solution that distills at 124.3 °C and contains 47.6% HBr by weight, which is 8.89 mol/L. Hydrobromic acid has a pKa of −9, making it a...
or phosphorus tribromide
Phosphorus tribromide
Phosphorus tribromide is a colourless liquid with the formula PBr3. It fumes in air due to hydrolysis and has a penetrating odour. It is widely used in the laboratory for the conversion of alcohols to alkyl bromides.-Preparation:...
(PBr3). A catalytic amount of may be used for the transformation using phosphorus and bromine; is formed in situ.
Iodoalkanes may similarly be prepared using using red phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
and iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
(equivalent to phosphorus triiodide
Phosphorus triiodide
Phosphorus triiodide is an unstable red solid which reacts violently with water. It is a common misconception that PI3 is too unstable to be stored; it is, in fact, commercially available. It is widely used in organic chemistry for converting alcohols to alkyl iodides. It is also a powerful...
). The Appel reaction
Appel reaction
The Appel reaction is an organic reaction that converts an alcohol into an alkyl chloride using triphenylphosphine and carbon tetrachloride. The use of carbon tetrabromide or bromine as a halide source will yield alkyl bromides, whereas using methyl iodide or iodine gives alkyl iodides...
is also useful for preparing alkyl halides. The reagent is tetrahalomethane and triphenylphosphine
Triphenylphosphine
Triphenylphosphine is a common organophosphorus compound with the formula P3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature...
; the co-products are haloform and triphenylphosphine oxide
Triphenylphosphine oxide
Triphenylphosphine oxide is the chemical compound with the formula OP3. Often chemists abbreviate the formula by writing Ph3PO or PPh3O . This white crystalline compound is a common side product in reactions involving triphenylphosphine...
.
From carboxylic acids
Two methods for the synthesis of alkyl halides from carboxylic acidCarboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
s are the Hunsdiecker reaction
Hunsdiecker reaction
The Hunsdiecker reaction is the organic reaction of silver salts of carboxylic acids with halogens to give organic halides. It is an example of a halogenation reaction...
and the Kochi reaction
Kochi reaction
The Kochi reaction is an organic reaction for the decarboxylation of carboxylic acids to alkyl halides with lead tetraacetate and a lithium chloride or other lithium salts .The reaction is a variation of the Hunsdiecker reaction....
.
Biosynthesis
Many chloro and bromolkanes are formed naturally. The principal pathways involve the enzymes chloroperoxidase and bromoperoxidaseBromoperoxidase
Bromoperoxidases are enzymes that catalyse the bromination of hydrocarbons. The enzymes accomplish this reaction via the following reaction:Related chloroperoxidase enzymes effect chlorination....
.
Reactions
Haloalkanes are reactive towards nucleophileNucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
s. They are polar
Chemical polarity
In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in...
molecules: the carbon to which the halogen is attached is slightly electropositive where the halogen is slightly electronegative. This results in an electron deficient
Electron deficiency
Electron deficiency occurs when a compound has too few valence electrons for the connections between atoms to be described as covalent bonds. Electron deficient bonds are often better described as 3-center-2-electron bonds...
(electrophilic) carbon which, inevitably, attracts nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
s.
Substitution
Substitution reactionSubstitution reaction
In a substitution reaction, a functional group in a particular chemical compound is replaced by another group. In organic chemistry, the electrophilic and nucleophilic substitution reactions are of prime importance...
s involve the replacement of the halogen with another molecule—thus leaving saturated hydrocarbons, as well as the halogenated product. Alkyl halides behave as the R+ synthon
Synthon
A synthon is a concept in retrosynthetic analysis. It is defined as a structural unit within a molecule which is related to a possible synthetic operation. The term was coined by E.J. Corey...
, and readily react with nucleophiles.
Hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
, a reaction in which water breaks a bond, is a good example of the nucleophilic nature of halogenoalkanes. The polar bond attracts a hydroxide
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
ion, OH– (NaOH(aq) being a common source of this ion). This OH– is a nucleophile with a clearly negative charge, as it has excess electrons it donates them to the carbon, which results in a covalent bond between the two. Thus C–X is broken by heterolytic fission resulting in a halide ion, X–. As can be seen, the OH is now attached to the alkyl group, creating an alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
. (Hydrolysis of bromoethane, for example, yields ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
). Reaction with ammonia give primary amines.
Alkyl chlorides and bromides are readily substituted by iodide in the Finkelstein reaction
Finkelstein reaction
The Finkelstein reaction, named for the German chemist Hans Finkelstein , is an SN2 reaction that involves the exchange of one halogen atom for another...
. The alkyl iodides produced easily undergo further reaction. Sodium iodide
Sodium iodide
Sodium iodide is a white, crystalline salt with chemical formula NaI used in radiation detection, treatment of iodine deficiency, and as a reactant in the Finkelstein reaction.-Uses:Sodium iodide is commonly used to treat and prevent iodine deficiency....
is used thus as a catalyst.
Alkyl halides react with ionic nucleophiles (e.g. cyanide
Cyanide
A cyanide is a chemical compound that contains the cyano group, -C≡N, which consists of a carbon atom triple-bonded to a nitrogen atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic....
, thiocyanate
Thiocyanate
Thiocyanate is the anion [SCN]−. It is the conjugate base of thiocyanic acid. Common derivatives include the colourless salts potassium thiocyanate and sodium thiocyanate. Organic compounds containing the functional group SCN are also called thiocyanates...
, azide
Azide
Azide is the anion with the formula N3−. It is the conjugate base of hydrazoic acid. N3− is a linear anion that is isoelectronic with CO2 and N2O. Per valence bond theory, azide can be described by several resonance structures, an important one being N−=N+=N−...
); the halogen is replaced by the respective group. This is of great synthetic utility: alkyl chlorides are often inexpensively available. For example, after undergoing substitution reactions, alkyl cyanides may be hydrolyzed to carboxylic acids, or reduced to primary amines using lithium aluminium hydride
Lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
. Alkyl azides may be reduced to primary alkyl amines by the Staudinger reduction or lithium aluminium hydride
Lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
. Amines may also be prepared from alkyl halides in amine alkylation
Amine alkylation
Amine alkylation is a type of organic reaction between an alkyl halide and ammonia or an amine. The reaction is called nucleophilic aliphatic substitution , and the reaction product is a higher substituted amine...
, the Gabriel synthesis
Gabriel synthesis
The Gabriel synthesis is named for the German chemist Siegmund Gabriel. Traditionally, it is a chemical reaction that transforms primary alkyl halides into primary amines using potassium phthalimide....
and Delepine reaction
Delepine reaction
The Delépine reaction is the organic synthesis of primary amines by reaction of a benzyl or alkyl halides with hexamethylenetetramine followed by acid hydrolysis of the quaternary ammonium salt...
, by undergoing nucleophilic substitution with potassium phthalimide
Potassium phthalimide
Potassium phthalimide is a chemical compound of formula C8H4KNO2. It is commercially available, and usually presents as fluffy, very pale yellow crystals. It is the potassium salt of phthalimide...
or hexamine
Hexamine
Hexamethylenetetramine is a heterocyclic organic compound with the formula 6N4. This white crystalline compound is highly soluble in water and polar organic solvents. It has a cage-like structure similar to adamantane. It is useful in the synthesis of other chemical compounds, e.g. plastics,...
respectively, followed by hydrolysis.
In the presence of a base, alkyl halides alkylate
Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion or a carbene . Alkylating agents are widely used in chemistry because the alkyl group is probably the most common group encountered in...
alcohols, amines, and thiols to obtain ether
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
s, N-substituted amines, and thioethers respectively. They are substituted by Grignard reagents to give magnesium salts and an extended alkyl compound.
Mechanism
Where the rate-determining step of a nucleophilic substitution reaction is unimolecular, it is known as an SN1 reactionSN1 reaction
The SN1 reaction is a substitution reaction in organic chemistry. "SN" stands for nucleophilic substitution and the "1" represents the fact that the rate-determining step is unimolecular...
. In this case, the slowest (thus rate-determining step) is the heterolysis of a carbon-halogen bond to give a carbocation and the halide anion. The nucleophile (electron donor) attacks the carbocation to give the product.
SN1 reactions are associated with the racemization
Racemization
In chemistry, racemization refers to the converting of an enantiomerically pure mixture into a mixture where more than one of the enantiomers are present...
of the compound, as the trigonal planar carbocation may be attacked from either face. They are favored mechanism for tertiary alkyl halides, due to the stabilization of the positive charge on the carbocation by three electron-donating alkyl groups. They are also preferred where the substituents are sterically bulky, hindering the SN2 mechanism.
Elimination
Rather than creating a molecule with the halogen substituted with something else, one can completely eliminateElimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
both the halogen and a nearby hydrogen, thus forming an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
by dehydrohalogenation
Dehydrohalogenation
Dehydrohalogenation is an organic reaction from which an alkene is obtained from an alkyl halide . It is also called a β-Elimination reaction and is a type of elimination reaction....
. For example, with bromoethane
Bromoethane
Bromoethane, also known as ethyl bromide, is a chemical compound of the haloalkanes group. It is abbreviated by chemists as EtBr. This volatile compound has an ether-like odour.-Preparation:...
and sodium hydroxide (NaOH) in ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
, the hydroxide
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
ion HO- abstracts a hydrogen atom. Bromide
Bromide
A bromide is a chemical compound containing bromide ion, that is bromine atom with effective charge of −1. The class name can include ionic compounds such as caesium bromide or covalent compounds such as sulfur dibromide.-Natural occurrence:...
ion is then lost, resulting in ethylene
Ethylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
), H2O and NaBr. Thus, haloalkanes can be converted to alkenes. Similarly, dihaloalkanes can be converted to alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
s.
In related reactions, 1,2-dibromocompounds are debrominated by zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
dust to give alkenes and geminal
Geminal
In chemistry, the term geminal refers to the relationship between two functional groups that are attached to the same atom...
dihalides can react with strong bases to give carbene
Carbene
In chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
s.
Other
Alkyl halides undergo free-radical reactions with elemental magnesium to give alkylmagnesium compounds: Grignard reagents. Alkyl halides also react with lithiumLithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
metal to give organolithium compounds. Both Grignard reagents and organolithium compounds behave as the R- synthon. Alkali metals such as sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
and lithium
Lithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
are able to cause alkyl halides to couple in the Wurtz reaction
Wurtz reaction
The Wurtz reaction, named after Charles-Adolphe Wurtz, is a coupling reaction in organic chemistry, organometallic chemistry and recently inorganic main group polymers, whereby two alkyl halides are reacted with sodium to form a new carbon-carbon bond:...
, giving symmetrical alkanes. Alkyl halides, especially iodides, also undergo oxidative addition
Oxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre...
reactions to give organometallic compounds.
Applications
Haloalkanes are widely used as synthonSynthon
A synthon is a concept in retrosynthetic analysis. It is defined as a structural unit within a molecule which is related to a possible synthetic operation. The term was coined by E.J. Corey...
equivalents to alkyl cation (R+) in organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
. They can also participate in a wide variety of other organic reactions.
Short chain haloalkanes such as dichloromethane
Dichloromethane
Dichloromethane is an organic compound with the formula CH2Cl2. This colorless, volatile liquid with a moderately sweet aroma is widely used as a solvent. Although it is not miscible with water, it is miscible with many organic solvents...
, trichloromethane (chloroform) and tetrachloromethane are commonly used as hydrophobic solvents in chemistry. They were formerly very common in industry, however, their use has been greatly curtailed due to their toxicity and negative environmental effects.
Chlorofluorocarbons were used almost universally as refrigerants and propellants due to their relatively low toxicity and high heat of vaporization. Starting in the 1980s, as their contribution to ozone depletion
Ozone depletion
Ozone depletion describes two distinct but related phenomena observed since the late 1970s: a steady decline of about 4% per decade in the total volume of ozone in Earth's stratosphere , and a much larger springtime decrease in stratospheric ozone over Earth's polar regions. The latter phenomenon...
became known, their use was increasingly restricted, and they have now largely been replaced by HFCs.

